Question
Question: Which of the following is an example of an aldotriose? A. Glyceraldehyde B. Ribose C. Fructose...
Which of the following is an example of an aldotriose?
A. Glyceraldehyde
B. Ribose
C. Fructose
D. Erythrose
Solution
An aldotriose is a molecule with an aldehyde group and which has three carbon atoms (triose). Therefore, first we have to draw the structures of each of the compounds mentioned. We can then count the number of carbon atoms and check if they are equal to three. Then we have to check for the presence of an aldehyde group. If both these conditions are satisfied, the chosen compound will be an aldotriose.
Complete step by step answer:
Let us first look at the structure of glyceraldehyde:
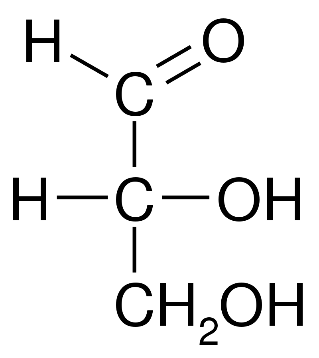
As we can see from the structure, this compound contains an aldehyde group as well as three carbon atoms. Therefore, it is an aldotriose.
Now let us look at the structure of ribose:
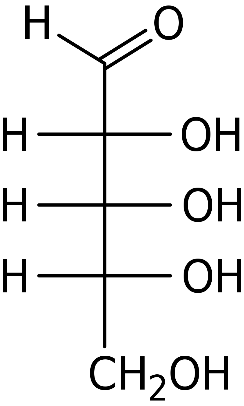
As we can see, ribose too has an aldehyde group, but it has a total of five carbon atoms. Thus, it cannot be considered as an aldotriose
We will now look into the structure of fructose:
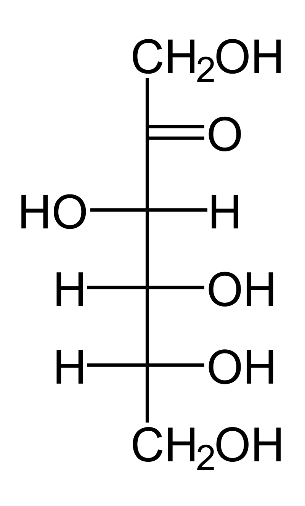
We see that not only does fructose not have an aldehyde group, it also has more than three carbon atoms. Hence, it cannot be considered as an aldotriose.
The last compound which we have is erythrose, whose structure is given below:
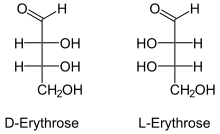
Although erythrose has an aldehyde group, it also has four carbon atoms, and thus, it cannot be an aldotriose.
Hence, as we have seen, glyceraldehyde is the only aldotriose from the options given to us.
So, the correct answer is Option A .
Note: In reality, there are just two trioses known to us, dihydroxyacetone and the two enantiomers of glyceraldehyde. Thus, the only identified aldotriose till now is glyceraldehyde.
The suffix ‘ose’ is usually used when referring to monosaccharide sugars. Ribose is thus, a pentose sugar while fructose and its isomer glucose are known as hexose sugars.
Note that each of these compounds are optically active due to the presence of a chiral carbon atom, and thus have different enantiomers.
