Question
Question: Which of the following is an electrophile? (A). \({H_2}O\) (B). \(S{O_3}\) (C). \(N{H_3}\) (...
Which of the following is an electrophile?
(A). H2O
(B). SO3
(C). NH3
(D). ROR
Solution
Electron loving species are known as electrophile. They do not contain any lone-pair on the central element and accept the lone-pair of other elements easily. Hence they act as Lewis acid.
Complete step by step answer:
As we know that are two types of species on the basis of electron count on an atom i.e. Nucleophile and Electrophile.
A.Nucleophile: Nucleophiles are known as nucleus loving chemical species, since the nucleus of an atom is positively changed. Therefore nucleophiles must be electron-rich chemical species containing at least one lone-pair of electrons. They may be either negatively charged and neutral nucleophiles contain at least one unshared pair of electrons, they have a strong tendency to donate this pair of electrons to electron deficient species and hence behave as Lewis bases E.g. H2O,NH3,R−OH,R−NH2 etc.
B.Electrophile: Electrophile are known as electron loving species. Their attraction for electrons is due to the presence of an electron deficient atom in them. Electrophile may be either positively charged or electrically neutral chemical species. Electrophile has a strong tendency to attract electrons and hence behave as Lewis acid.
E.g. SO3,BF3,AlCl3 etc.
Now the structure of given compound is as follow:
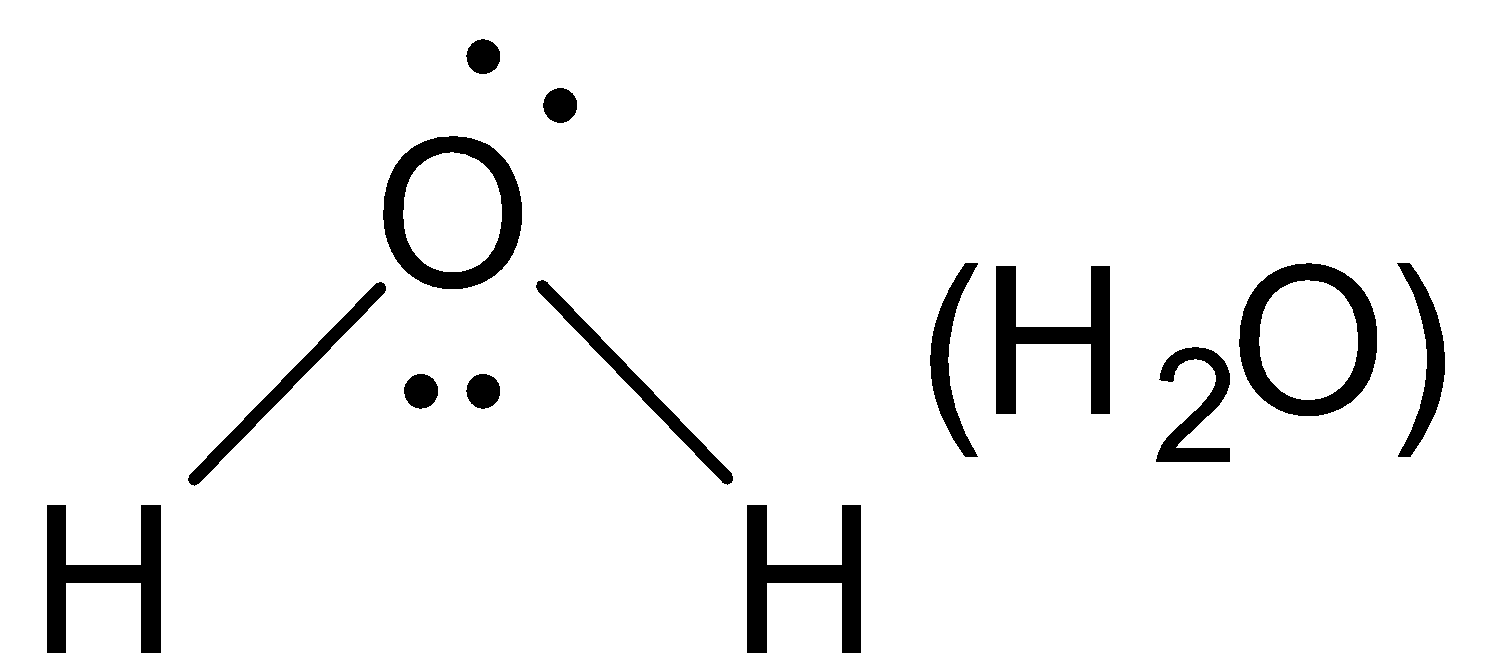
Central atom contains two lone pairs. Thus it is a nucleophile.
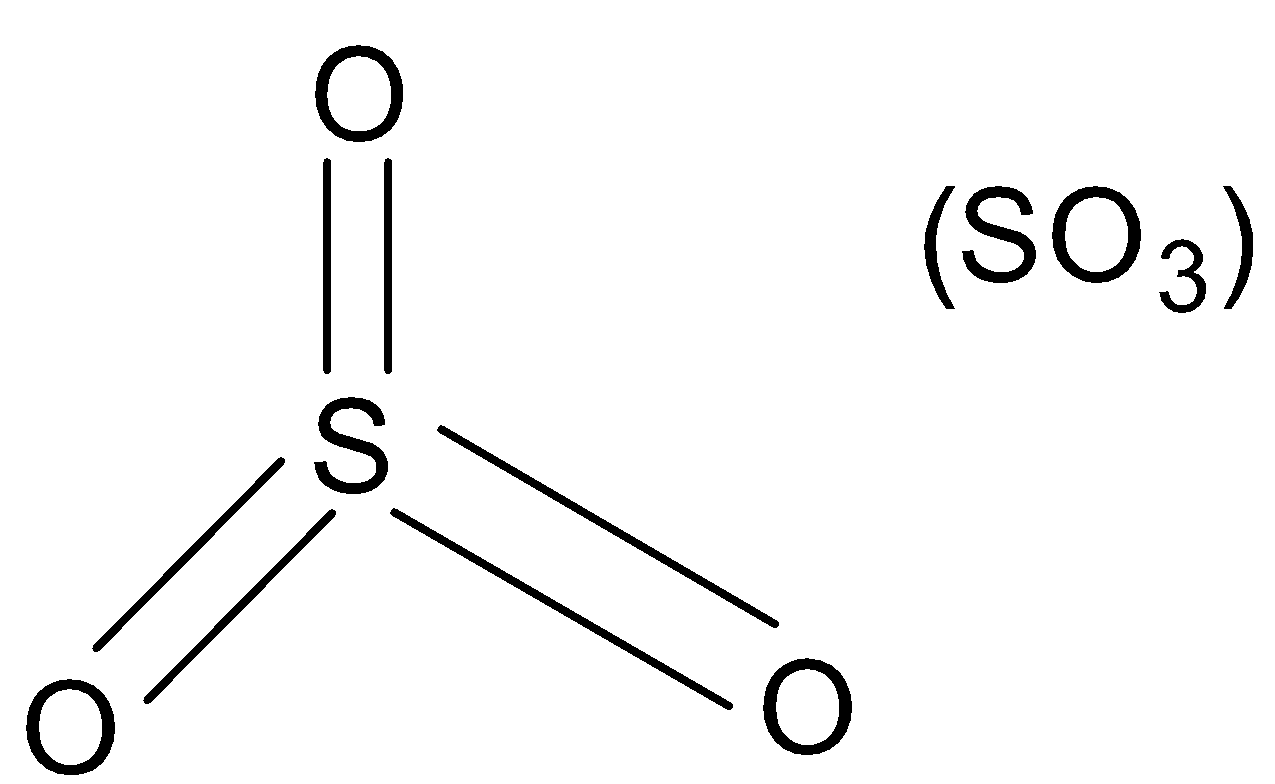
No electron/lone-pair present. Thus it is an electrophile.
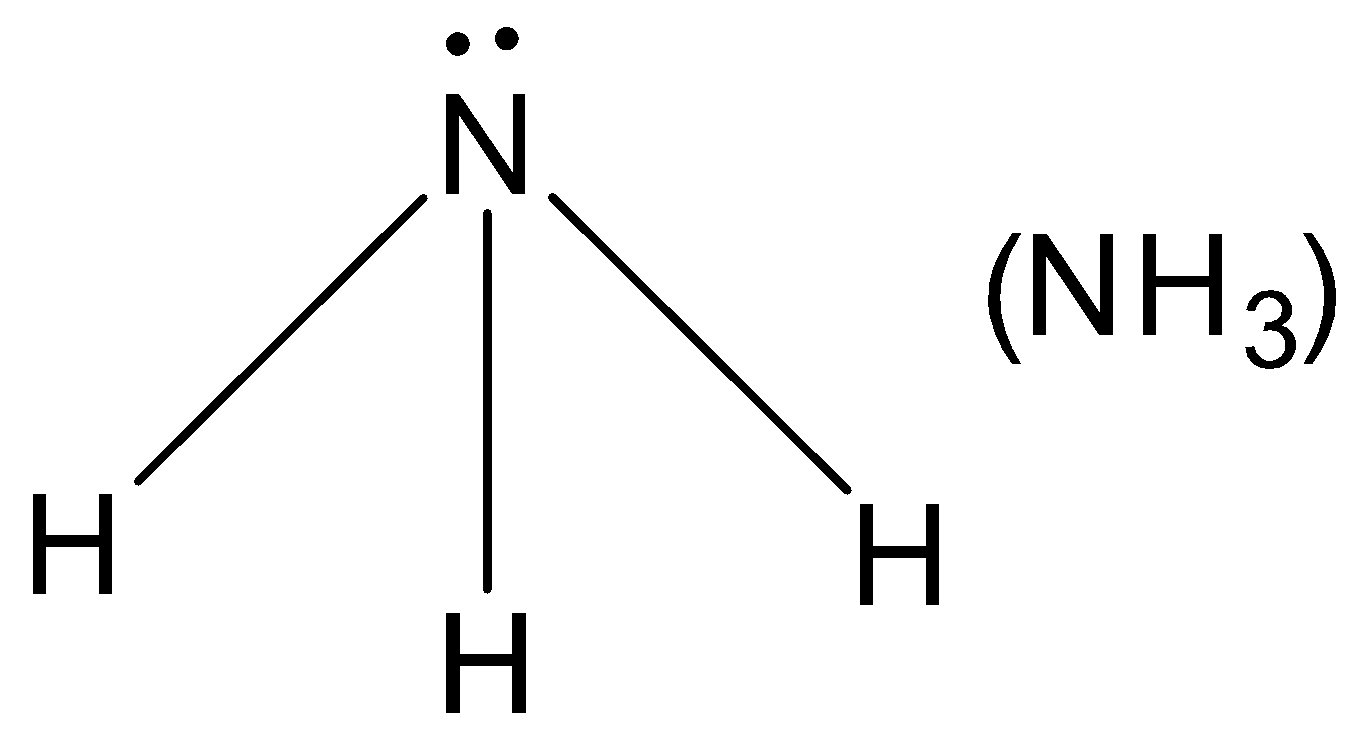
Central atom N contains one lone-pair. Thus it is a nucleophile.
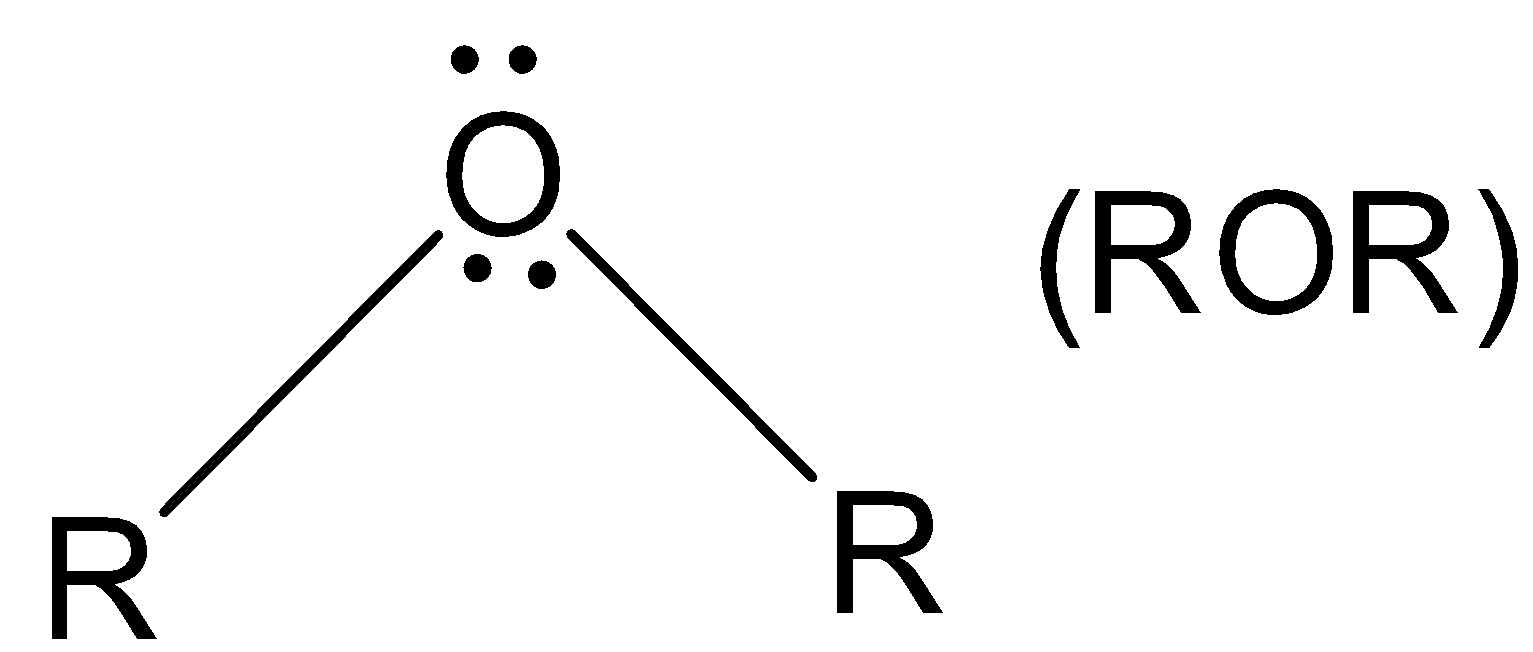
Central atom contains two lone pairs and thus it is a nucleophile.
So we get to know that out of a given compound only SO3 does not contain electrophile and thus is an electrophile.
So, the correct answer is Option B.
Note: In SO3 molecule, electrons and lone-pairs are present on the oxygen atoms but not on the sulphur atom. So it is an electrophile. But in any case if it gets bonded through an oxygen atom to another compound it will behave as a nucleophile.
