Question
Question: Which of the following is an electrophile? A. \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] B. \[{\te...
Which of the following is an electrophile?
A. :CCl2
B. CH3 -
C. H2O
D. NH3
Solution
Electrophile is an electron-deficient species. It tends to accept electrons. Determine the species that has less number of electrons.
Complete answer:
An electrophile is a species having an electron-deficient atom or center. Due to the deficiency of electrons, they tend to gain electrons.
A. The species given in option A is:CCl2. It is dichlorocarbene. The structure of :CCl2 is as follows:
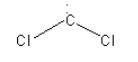
Here, the central carbon atom is surrounded by 6 electrons so we can say that it is an electron-deficient species. So, dichlorocarbene :CCl2 is an electrophile.
Thus, option (A) :CCl2 is correct.
B. The species given in option B isCH3 - . The structure of CH3 - is as follows:
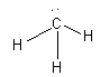
Here, the central carbon atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider CH3 - as an electrophile.
Thus, option (B) CH3 - is incorrect.
C. The species given in option C isH2O. The structure of H2O is as follows:
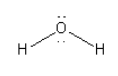
Here, the central oxygen atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider H2O as an electrophile.
Thus, option (C) H2O is incorrect.
D. The species given in option D isNH3. The structure of NH3 is as follows:
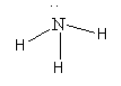
Here, the central nitrogen atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider NH3 as an electrophile.
Thus, option (D) NH3is incorrect.
**Hence, the correct option is (A) :CCl2.
Note:**
Carbene are neutral species having a carbon atom with two bonds. In carbene central carbon atoms are surrounded by 6 electrons. As the octet of central carbon in carbene is incomplete they are known as electrophile.
