Question
Question: Which of the following is an electron-deficient compound? \[ A.{\text{ N}}{{\text{H}}_{\text{3...
Which of the following is an electron-deficient compound?
A. NH3 B. ICl C. BCl3 D. PCl3Solution
Hint : We must know that the electron deficient compound is the one in which there are not enough electrons to completely fill the outer shell (octet) of the central atom.
Complete step by step solution :
Let’s first discuss Electron deficient compounds. Electron deficient compound is the one in which there are not enough electrons to completely fill the outer shell (octet) of the central atom. These compounds contain less number of electrons i.e. they are electron deficient to form normal electron-pair bonds between each pair of atoms.
So, looking at the options provided in the question let’s check which compound’s central atom is not having its octet complete.
Case 1
In case of NH3the electron dot structure will be
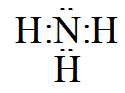
Clearly, the octet of nitrogen is completely filled. (Valence electron of nitrogen and hydrogen are 5 and 1 respectively)
Case 2
In case of ICl the electron dot structure will be like
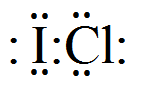
Clearly, the octet of both the atoms is completely filled. (Valence electrons of Iodine and Chlorine are 7 each)
Case 3
In case of BCl3the electron dot structure will be like
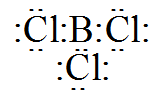
In this it is clearly visible that the central atom is not having a complete octet so it will be an electron deficient compound. (Valence electrons of Boron and Chlorine are 3 and 7 respectively)
Case 4
In case of PCl3 the electron dot structure will be like
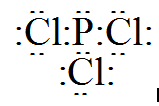
Clearly, the octet of the central atom is complete. (Valence electron of phosphate and chlorine is 5 and 7 respectively)
So, the answer to this question is C. BCl3.
Note : We must know that Lewis dot structure or we can say electron dot structures are diagrams that represent the valence electrons of an atom in a molecule. These structures help us visualize the valence electron of an atom in a molecule. Also tell us whether these valence electrons exist in bonds or lone pairs.
