Question
Question: Which of the following is an anhydride ? (A) 
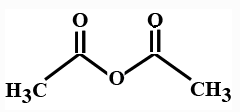
(B)
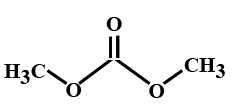
(C)
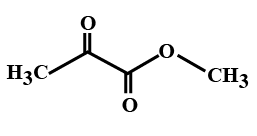
(D)
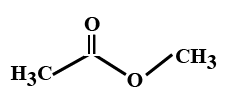
Solution
Hint : We can define an anhydride compound as a compound that is formed from another compound by dehydration or elimination of water. Anhydrides have functional groups which used to be derivatives of either acid or bases. Basic anhydrides have a different pattern from acid anhydrides. Anhydrides can be organic and inorganic both.
Complete step by step solution:
In organic chemistry acid anhydrides contain the functional group R(CO)O(CO)R′. Hence an acid anhydride compound contains two acyl groups bonded to the same oxygen atom. A common example of acid anhydride is carboxylic anhydride whose parent acid is a carboxylic acid. The organic anhydrides introduces the acyl group (RCO) in organic synthesis. Anhydrides reacts with water and produces carboxylic acids.
In the options all are organic compounds. In the given organic compounds we have to identify the functional group R(CO)O(CO)R′. If in the given compounds we consider CH3− group as R− group then analysis will become easy. In the first option we have found this compound satisfy the formulaR(CO)O(CO)R′ of anhydride compounds where R=R′=CH3, hence this is a symmetric type of anhydride. The rest of the option does not satisfy the chemical formula of anhydrides so they are not anhydrides.
So here option A is the correct answer to this question. Compound ( A) is acetic anhydride.
Note : Anhydrides produces ester when it reacts with alcohol or phenol and with ammonia and amines it produces amides. Symmetric acid anhydrides can be prepared easily and have a wide range of applications. These are used in synthesis of other useful organic compounds and used as reagents for amines.
