Question
Question: Which of the following is an allylic halide? A) 3-bromo-2-methylpropene B) 3-bromobut-1-ene C)...
Which of the following is an allylic halide?
A) 3-bromo-2-methylpropene
B) 3-bromobut-1-ene
C) 3-bromopent-2-ene
D) 4-bromobut-1-ene
Solution
. To answer this question, first you should know what allylic halides are. They are the molecules in which the halogen atom is attached to the carbon atom which is further attached to the carbon-carbon double bond. Draw the chemical structures of each compound given in options and examine their structure.
Complete step by step answer:
Allylic halides are the halides in which the halogen atom is bonded to sp3- hybridised carbon atom next to carbon-carbon double bond (C=C). General chemical structure of allylic halides:
CH2=CH−CH2−X
Here, X is any halogen atom.
Now, let us draw the structure of each given compound to find the allylic halide.
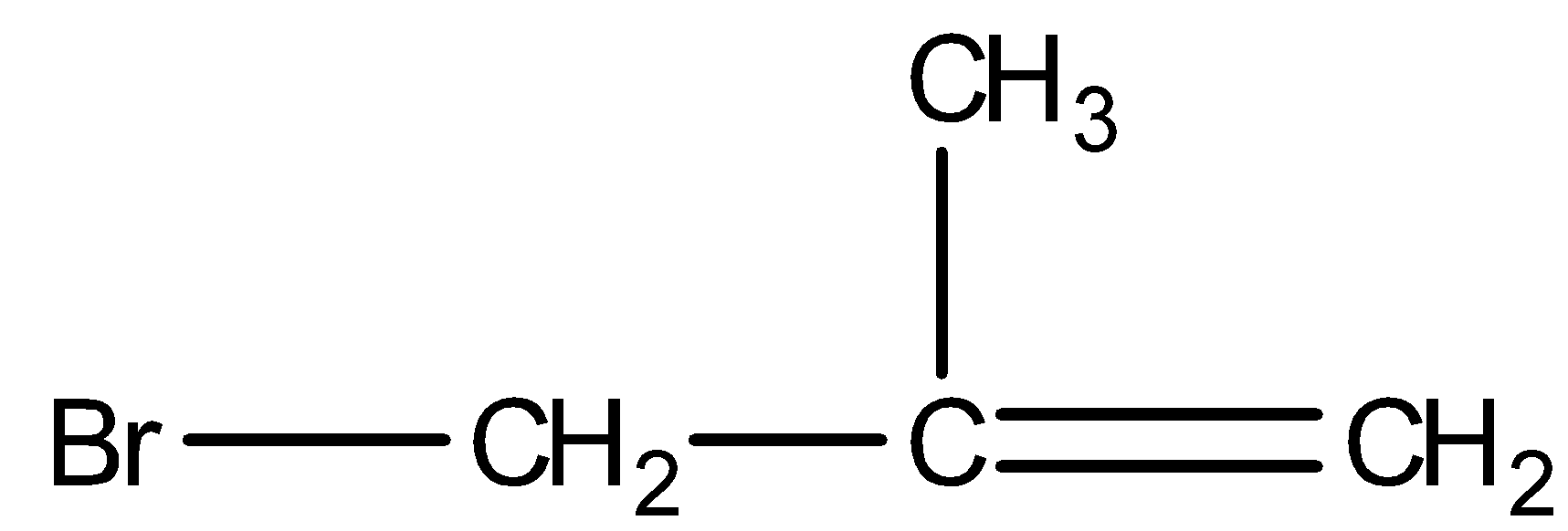
- 3-bromo-2-methylpropene:
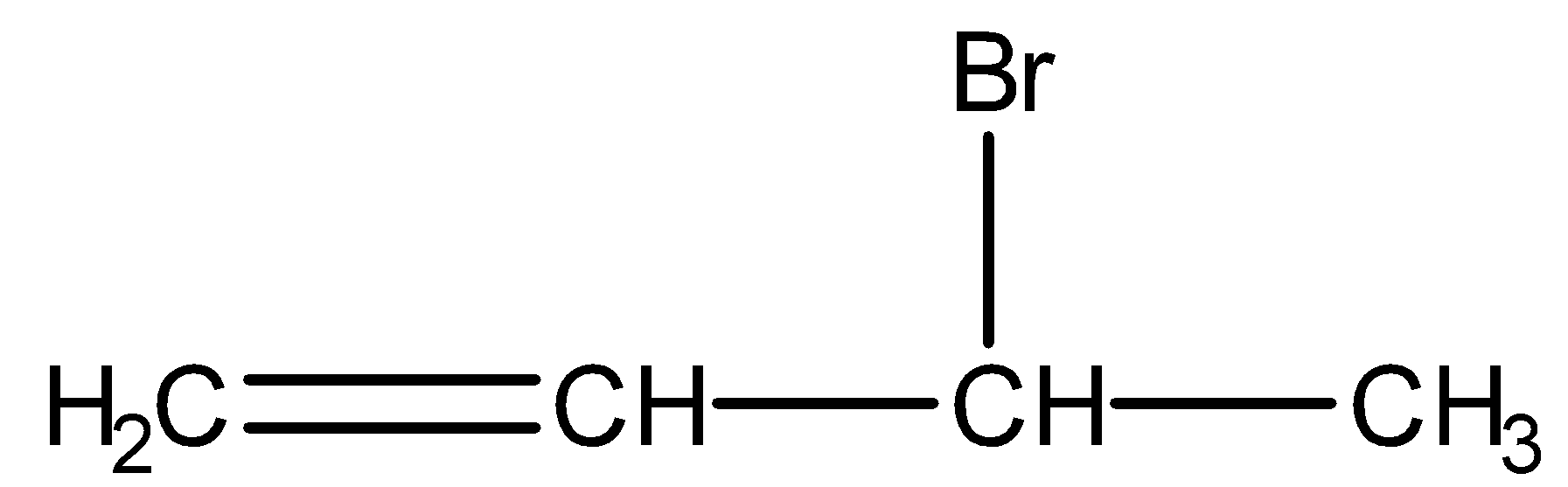
- 3-bromobut-1-ene:
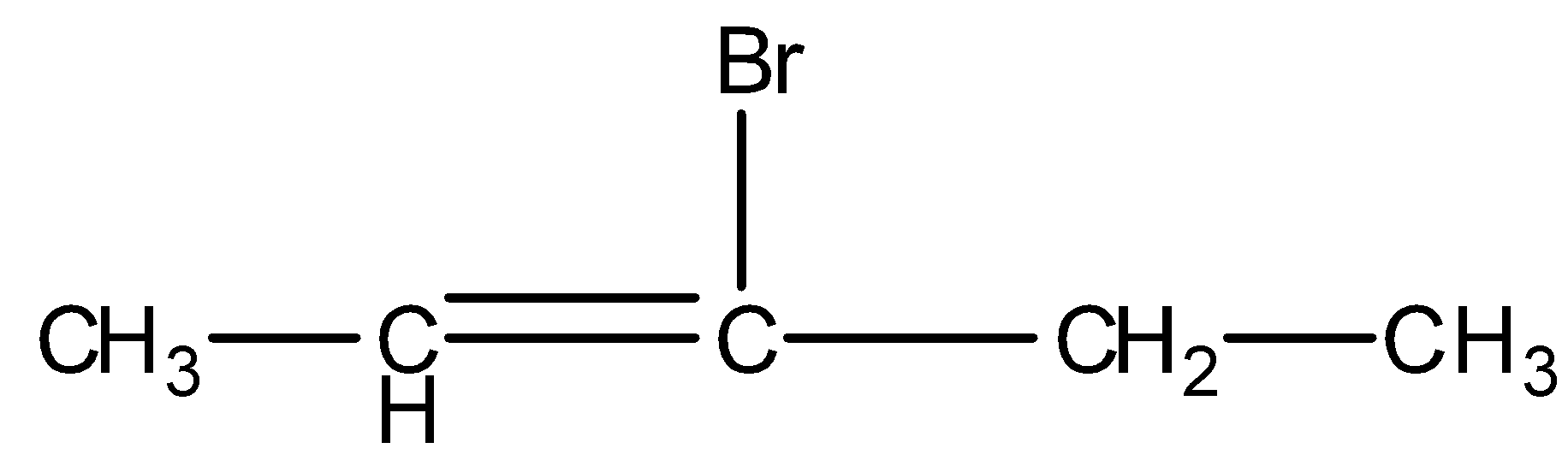
- 3-bromopent-2-ene
- 4-bromobut-1-ene
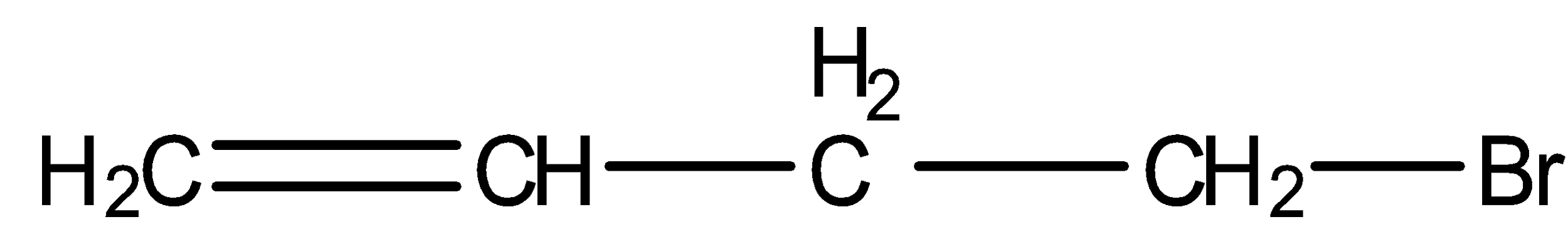
We can see from the above structures that in, 3-bromo-2-methylpropene and 3-bromobut-1-ene, bromine is attached to the sp3 -hybridised carbon atom which is next to carbon-carbon double bond or sp2 - hybridised carbon. Thus, both 3-bromo-2-methylpropene and 3-bromobut-1-ene are the allylic halides.
So, the correct answer is “Option A and B”.
Additional Information:
One can confuse between allylic halides and vinyl halides. You must know that vinyl halides are the compounds in which the halogen atom is bonded to the carbon atom that is doubly bonded to another carbon atom or we can simply say that, halogen atom is bonded to an sp2- hybridised carbon. General formula of vinyl halides is: CH2=CH2−X. Here, X is any halogen atom.
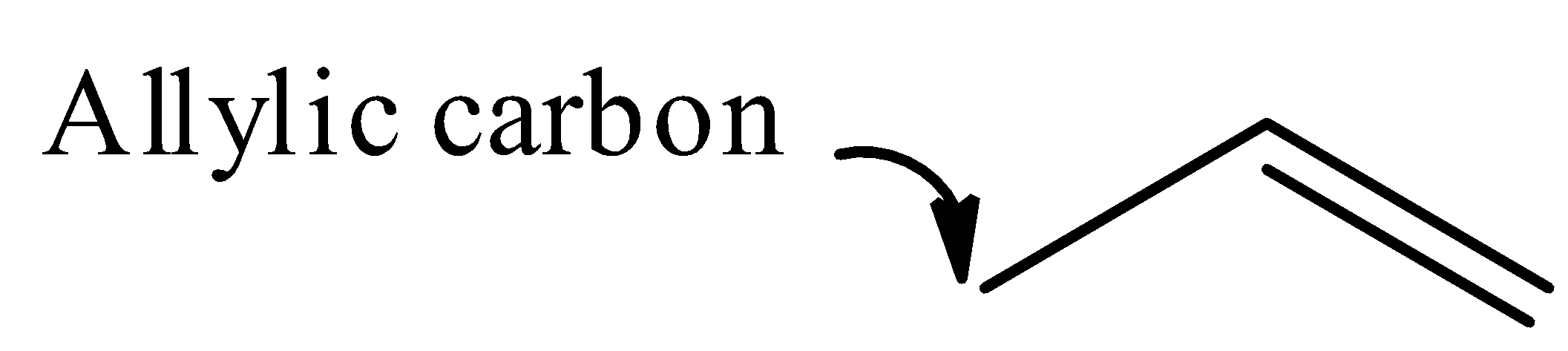
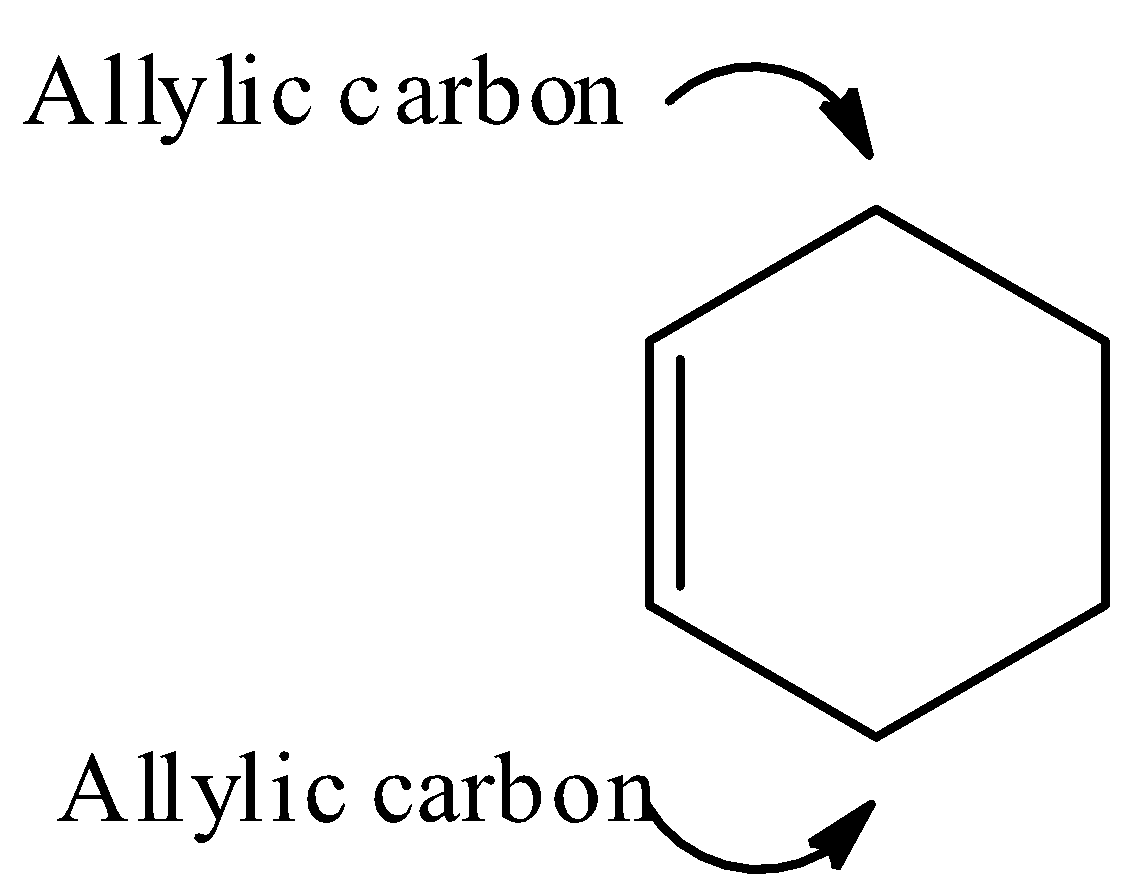
Note: It should be noted that the carbon atom bonded to carbon atom that in turn is doubly bonded to another carbon atom is known as allylic carbon. Examples of allylic carbon: