Question
Question: Which of the following is among the major products of the reaction of (E)-3-methy1,2-pentene with \(...
Which of the following is among the major products of the reaction of (E)-3-methy1,2-pentene with BH3 in THF followed by the addition of H2O2/OH− ?
A) 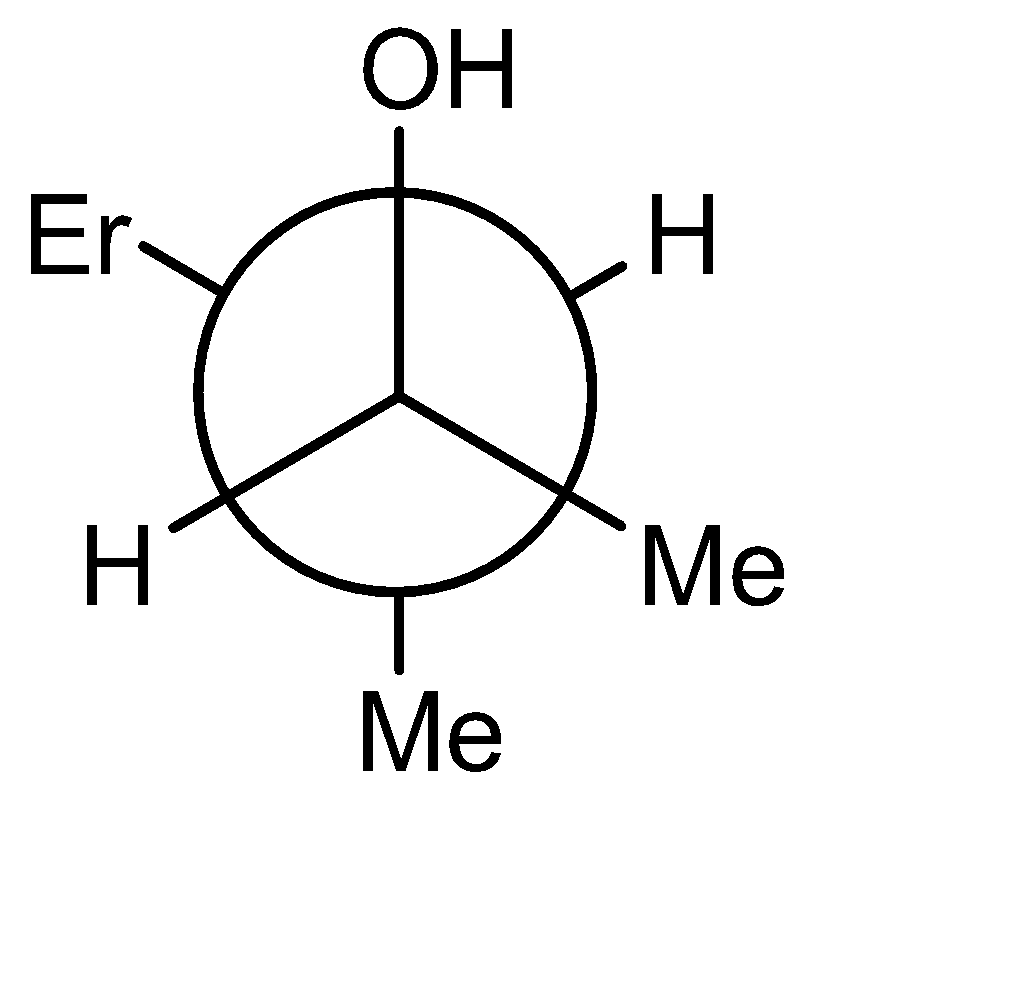
B) 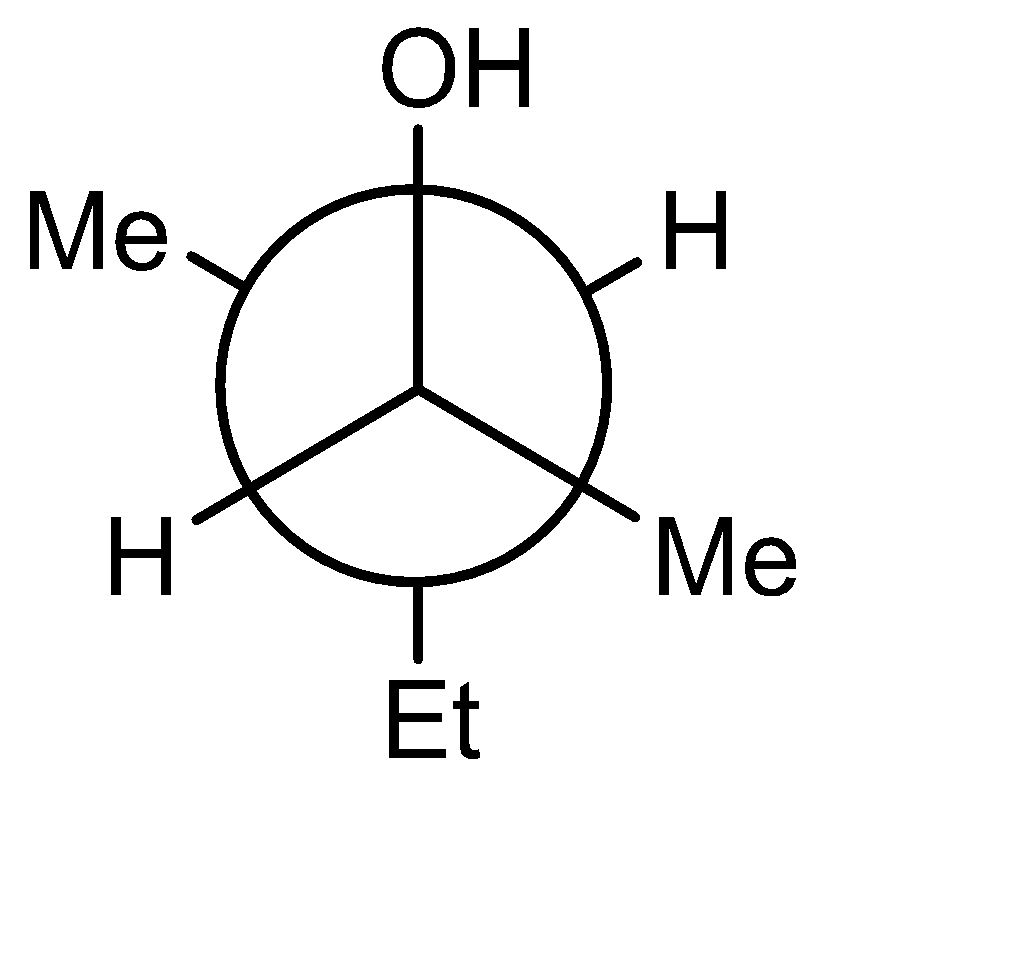
C) 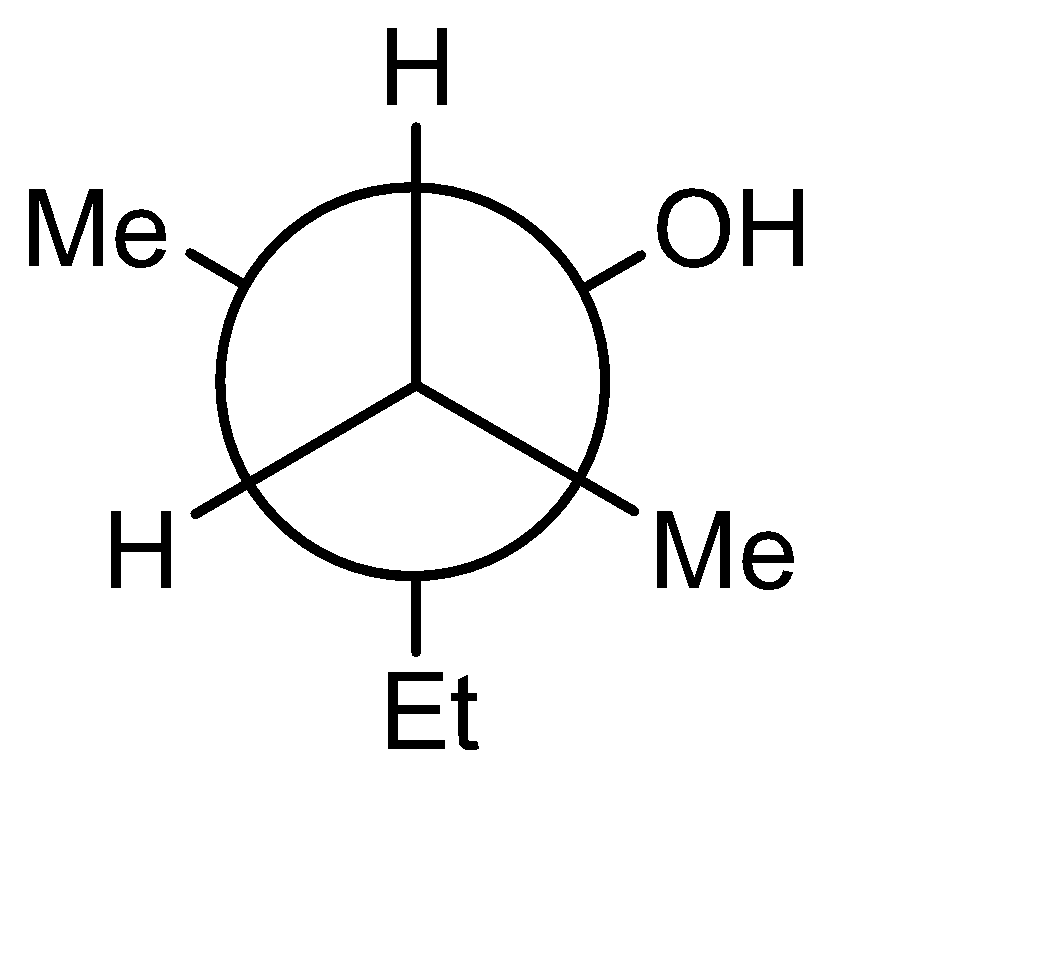
D) 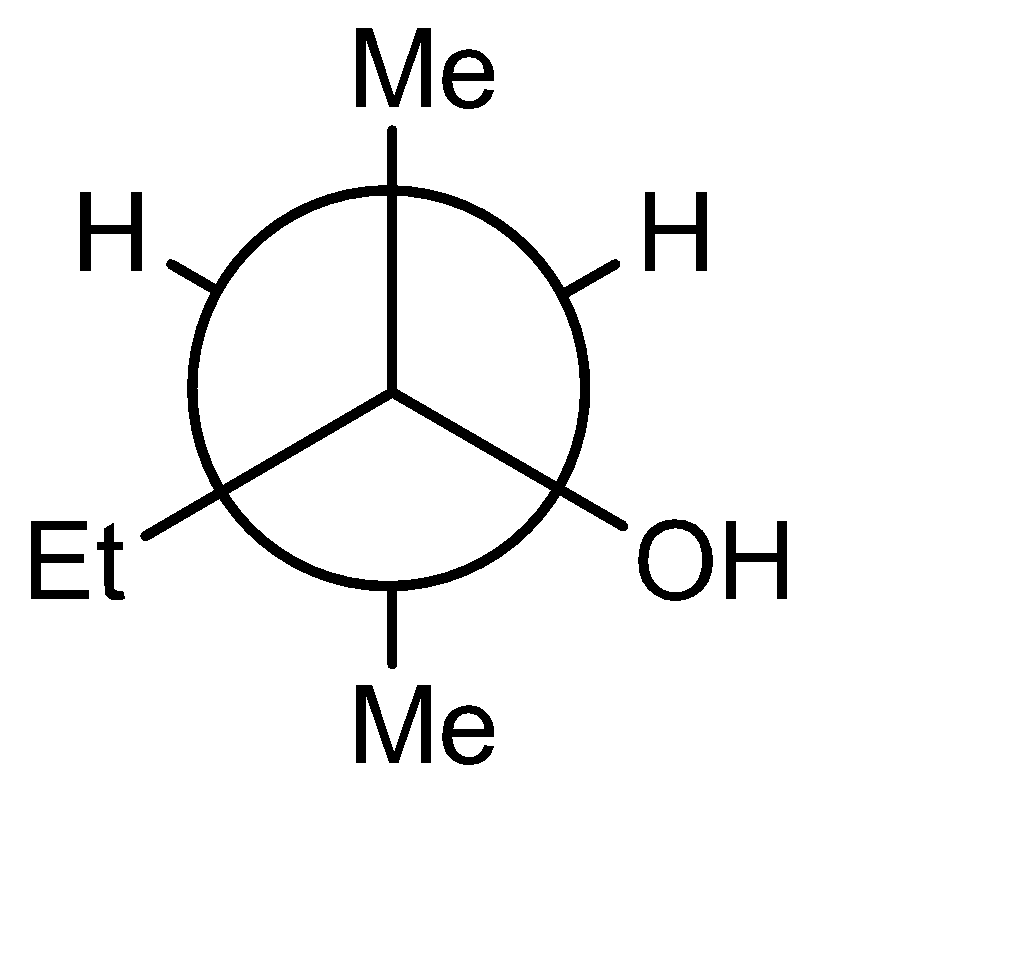
Solution
The process given to us is the Hydroboration-Oxidation process. The reagents are BH3 and H2O2/OH− which assures that it is hydroboration oxidation. This process is common for the conversion of Alkenes to Alcohols. To solve this question, we will look into stereochemistry of the reaction.
Complete answer:
Hydroboration-Oxidation is a common technique for the production of alcohols. The reaction is Anti-Markovnikov in nature (meaning the negative part of the reagent gets attached to that carbon atom having more no. of hydrogen). Here the hydrogen will get attached to the less substituted carbon and the boron will get attached to the more substituted carbon atom along the alkene bond. The borane given in the question acts as the anti-markovnikov Lewis acid. This process doesn’t require any catalyst to start. The mechanism of Hydroboration Oxidation has the characteristics of both hydrogenation and electrophilic substitution and the addition occurs in a ‘syn’ manner, meaning the hydroboration takes place in the same phase as that of the double bond, which leads to a cis stereochemistry. The general representation of the addition is shown below:
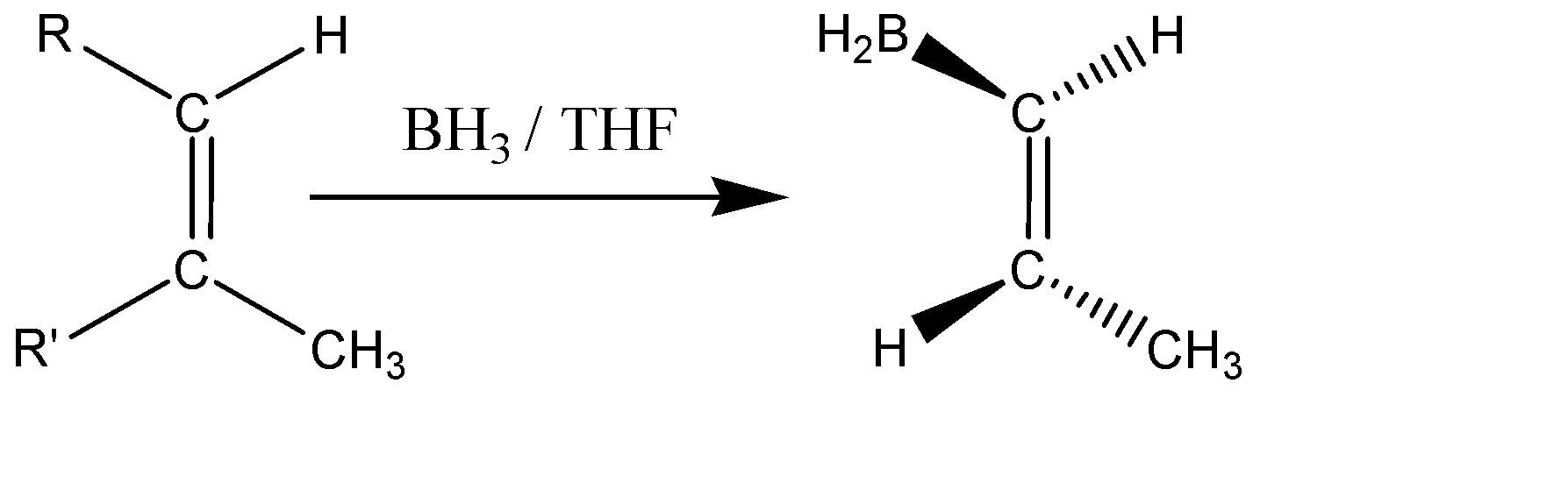
It is seen that the BH2&H are added in the same phase (above the plane). The addition is syn and is an Anti-markovnikov in nature.
Let us now predict the products of the given reaction of E)-3-methy1,2-pentene with BH3 in THF followed by the addition of H2O2/OH− . The reaction can be given as:
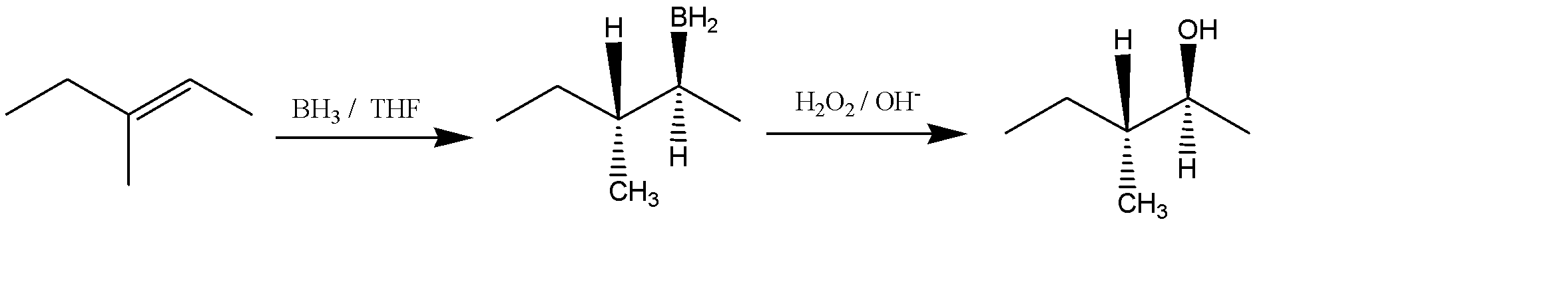
The product obtained is an alcohol (3-methyl pent-2-ol). The -OH and -H are above the plane. The Newman projection of the same will be formed as:
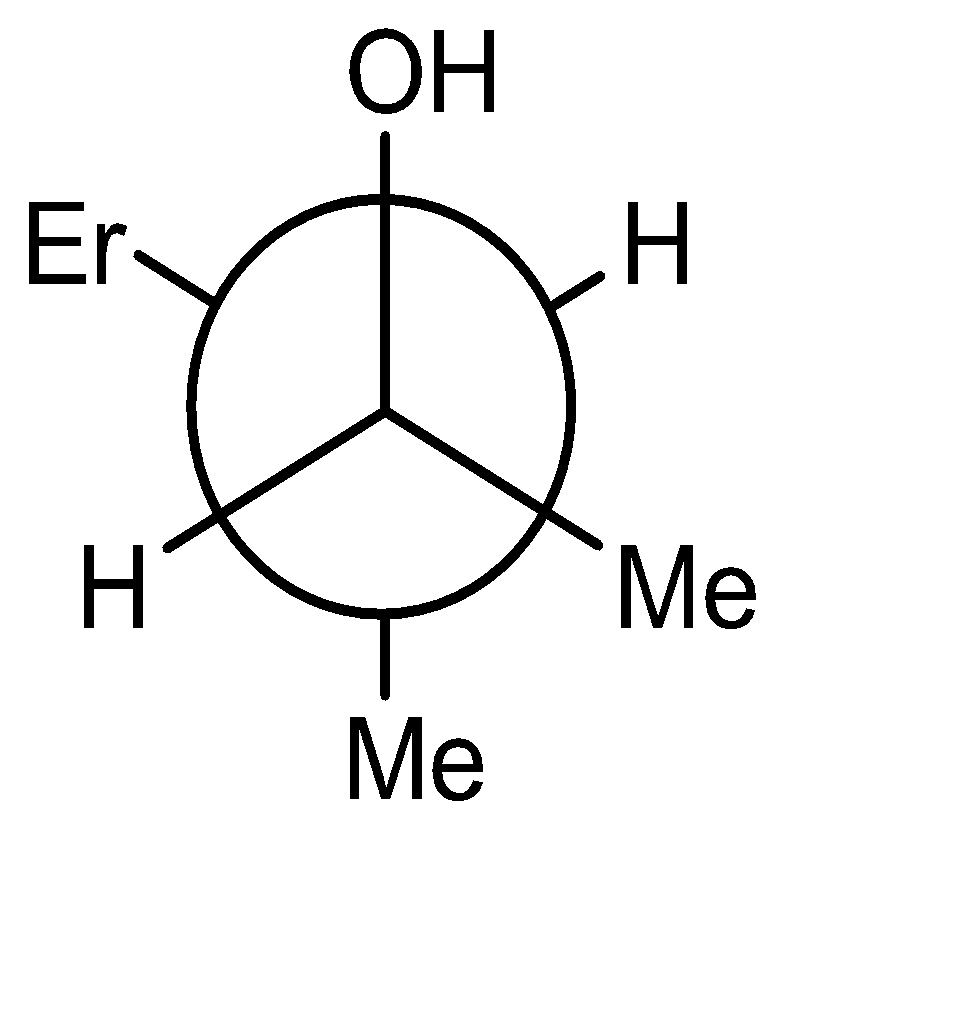
Hence the correct option is (A).
Note:
The intermediate formed in the reaction is known as organo-borane and can be easily converted to alcohol by using simple peroxides. If electron withdrawing substituents are present, the measure of anti-markovnikov addition reduces and the major product obtained thus is a Markovnikov product.
