Question
Question: Which of the following is allylic halide? (a) (1-Bromoethyl) benzene (b) Benzyl chloride (c) 1...
Which of the following is allylic halide?
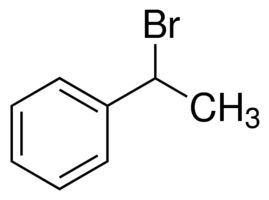
(a) (1-Bromoethyl) benzene
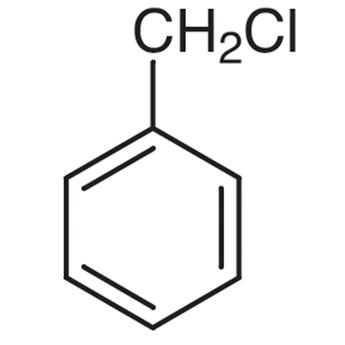
(b) Benzyl chloride
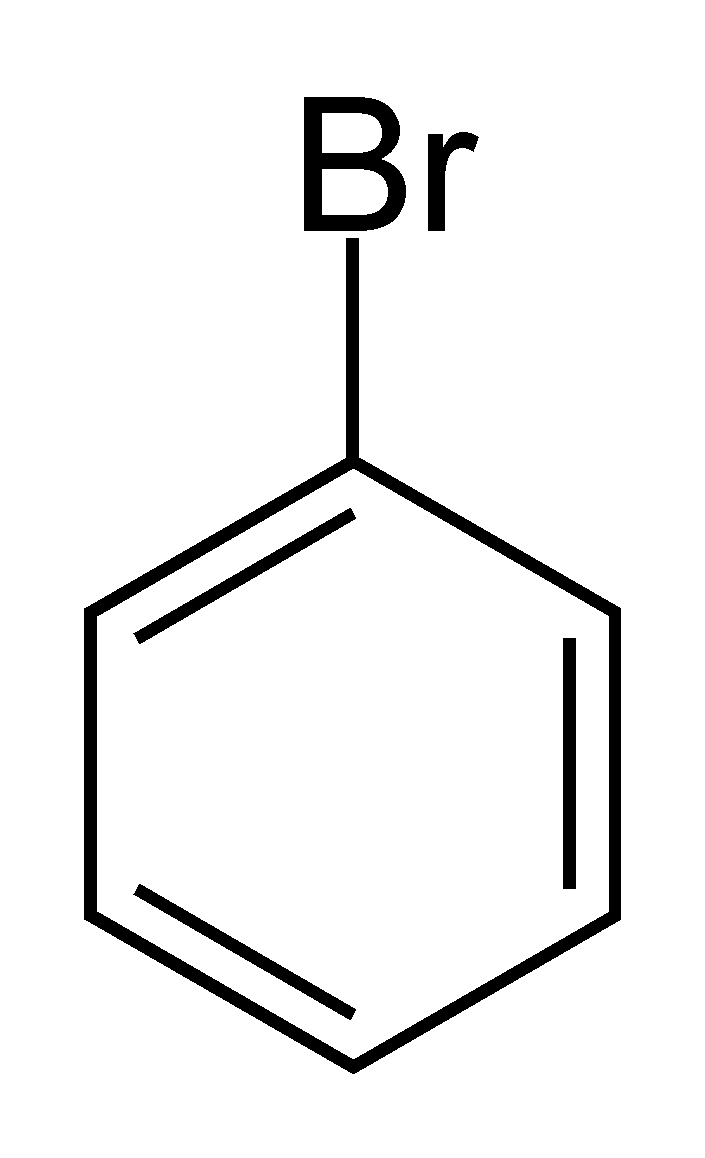
(c) 1-Bromo benzene
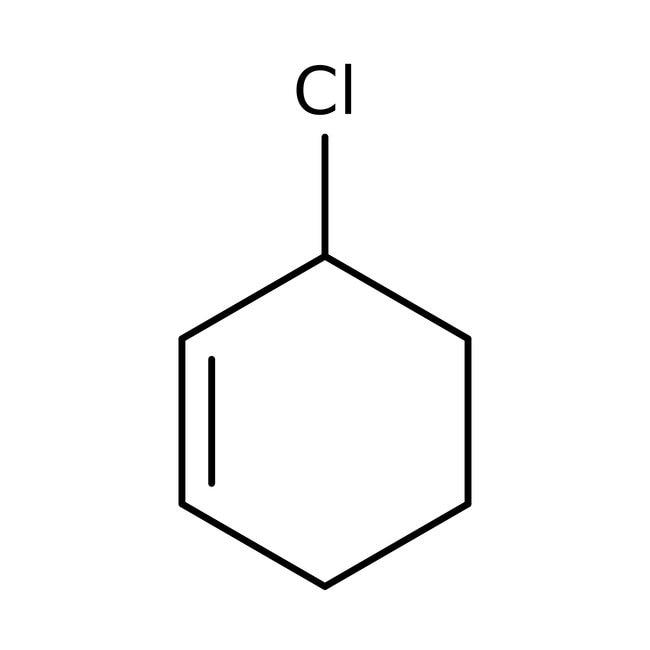
(d) 3-Chloro cyclohex-1-ene
Solution
Hint: An allylic carbon is a carbon atom bonded to a carbon atom that in turn is doubly bonded to another carbon atom.
Step-by-step answer :
In (1-Bromoethyl) benzene or C8H9Br, we can see that on the allylic carbon of the molecule, the halogen atom is present with a methyl group. Hence, it cannot be termed as an allylic halide.
(1-Bromoethyl) benzene
In Benzyl chloride or (Chloromethyl) Benzene with a molecular formula of C7H7Cl, we can see that on the allylic carbon of the molecule, the halogen atom is present along with a methyl group. This is why Benzyl chloride does not qualify as an allylic halide.
Benzyl chloride
1-Bromo benzene or C6H5Br as we can see does not have the halogen atom Br bonded to sp3−hybridised carbon atom next to the Carbon-Carbon double bond (C=C). This denotes that 1-Bromo benzene is not an allylic halide.
1-Bromo benzene
An allylic halide is an alkyl halide which has one or more halogen atoms on the allylic carbons of the molecule. In the below given figure of 3-Chloro cyclohex-1-ene, we can see a halogen atom bonded to sp3−hybridised carbon atom next to the Carbon- Carbon double bond (C=C). This denotes that 3-Chloro cyclohex-1-ene or C7H11Cl is allylic halide.
3-Chloro cyclohex-1-ene
So, we can figure out that Option D is the correct answer.
Note: Allylic halide is the one in which the carbon atom next to the double bonded carbon atom carries one or more halogen atoms. Allylic halides are reactive in both SN1 and SN2 mechanisms.