Question
Question: Which of the following is a tricarboxylic acid? (A)Citric acid (B)Malonic acid (C)Succinic aci...
Which of the following is a tricarboxylic acid?
(A)Citric acid
(B)Malonic acid
(C)Succinic acid
(D)Malic acid
Solution
As we know that carboxylic acid is an organic acid which contains a carboxyl group (C(=O)OH) attached to an R-group (R = alkyl or aryl). Tricarboxylic acid, by the name itself, says that it is a category of carboxylic acid which has 3 C(=O)OH group and hence we will draw the structure of each compound given in the options and check for 3 C(=O)OH group.
Complete step-by-step answer: -Carboxylic acid are one of the class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (―OH) by a single bond i.e., its functional group represented as C(=O)OH.
-Carboxylic acids occur widely in nature and its derivatives are of utmost importance in various chemical reactions.
-Tricarboxylic acids belongs to the class of carboxylic acids which contains 3 carboxyl groups (C(=O)OH) attached to R-groups (R = alkyl or aryl).
Now let us draw structure of each acid compound given in the options:-
(A)Citric acid:
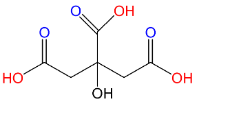
As we can see that this compound carries 3 carboxyl groups (C(=O)OH) attached to the parent chain. Hence this is a tricarboxylic acid. Citric acid is a weak acid which naturally occurs in citric fruits.
(B)Malonic acid:
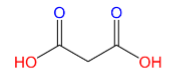
Here only 2 carboxyl groups (C(=O)OH) are present, therefore Malonic acid is a dicarboxylic acid.
(C)Succinic acid:
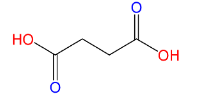
Here also only 2 carboxyl groups (C(=O)OH) are present, therefore Succinic acid is a dicarboxylic acid.
(D)Malic acid:
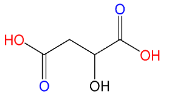
Here also only 2 carboxyl groups (C(=O)OH) are present, therefore Malic acid is a dicarboxylic acid.
Therefore the correct option is (A) Citric acid.
Note: -Carboxylic acids generally have higher melting point than other hydrocarbons and functional groups because of its intermolecular hydrogen bonding. And since Tricarboxylic acids have 3 carboxyl groups (C(=O)OH) attached to R-groups, it do have the ability to form strong hydrogen bonds and this results in their high boiling points.
-As the number of carboxyl group increases in the compound, nomenclature changes as follows:-
1 (C(=O)OH) group : monocarboxylic acid (or simply carboxylic acid)
2 (C(=O)OH) group : dicarboxylic acid
3 (C(=O)OH) group : tricarboxylic acid
4 (C(=O)OH) group : tetracarboxylic acid
and so on.
