Question
Question: Which of the following is a transition element? A. \(Al\) B.\(As\) C.\(Ni\) D.\(Rb\)...
Which of the following is a transition element?
A. Al
B.As
C.Ni
D.Rb
Solution
Transition elements are those elements which are placed between metals and non - metals in periodic table. They are also called d-block elements.
Complete step by step answer:
Let us first explain the periodic table.
According to modern periodic table given by Moslay:
Non - Metals are placed in group 13 to group 17.Inert gases are placed in group 18. And transition elements are placed in group 3 to group 12.
Aluminum(Al) belongs to group 3. Therefore, it is metal.
Arsenic(As) belongs to the group 15 therefore it is metalloid.
Rubidium (Rb) belongs to group 1. Therefore, it is metal.
Nickel (Ni) belongs to group 10, therefore it is transition metal.
So, the correct answer is “Option C”.
Additional information:
The Atomic Number of Ni is 28.
It’s electronic configuration is 1s22s22p63s23p63d84s2.
=[Ne]3s23p63d84s2.
Since Electronic configuration of Ne is 1s22s22p6. Therefore, we can write Ne in place of 1s22s22p6.
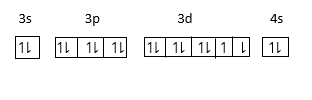
The energy of 4s orbital is less than 3d orbital therefore electron enters in 4s orbital first and then 3d orbital therefore electron enters in us first and then 3d. Thus last electron in Nickel enters in d− orbital. Therefore, it is known as d−block elements.
Note:
There are four transition series called as first (4d), second (5d), third (6d) and Fourth (7d). Nickel belongs to the first transition series.
Transition element can be identified by filling electrons in the orbital. If the last electron enters in d−orbital it is a transition element. Electrons should be filled according to Aufbau principle.
