Question
Question: Which of the following is a secondary alcohol? A. 2-methyl-2-propanol B. 1-propanol C. 1-butan...
Which of the following is a secondary alcohol?
A. 2-methyl-2-propanol
B. 1-propanol
C. 1-butanol
D. 2-pentanol
Solution
The alcohol functional group in organic compounds is going to be represented with a symbol –OH. As per the position of the alcohol functional group in the organic compounds alcohols are three types and they are primary alcohol () , secondary alcohol ( ) and tertiary alcohol ().
Complete answer:
- In the question it is asked to find the secondary alcohol among the given compounds in the question.
- If alcohol functional group is attached to primary carbon in an organic compound then the alcohol is called primary alcohol, if the alcohol is attached to secondary carbon then the alcohol is called secondary alcohol and if the alcohol functional group is attached to tertiary carbon then the alcohol is called tertiary alcohol.
- Without knowing the structures of the compounds in the given options we cannot find the secondary alcohol.
- Coming to the given options, the structure of 2-methyl-2-propanol in the option A is as follows.
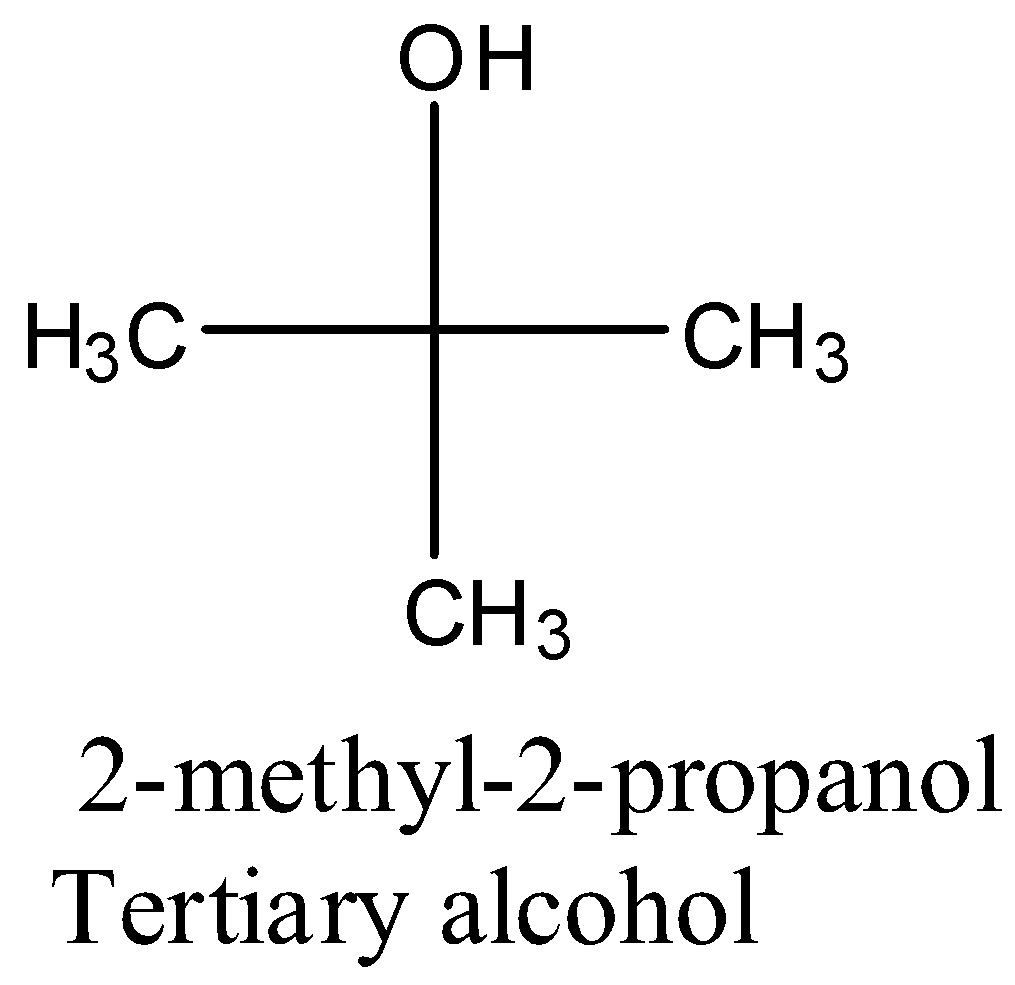
- The compound in option A is a tertiary alcohol.
- Coming to the option B, 1-propanol.
- The structure of 1-propanol is as follows.
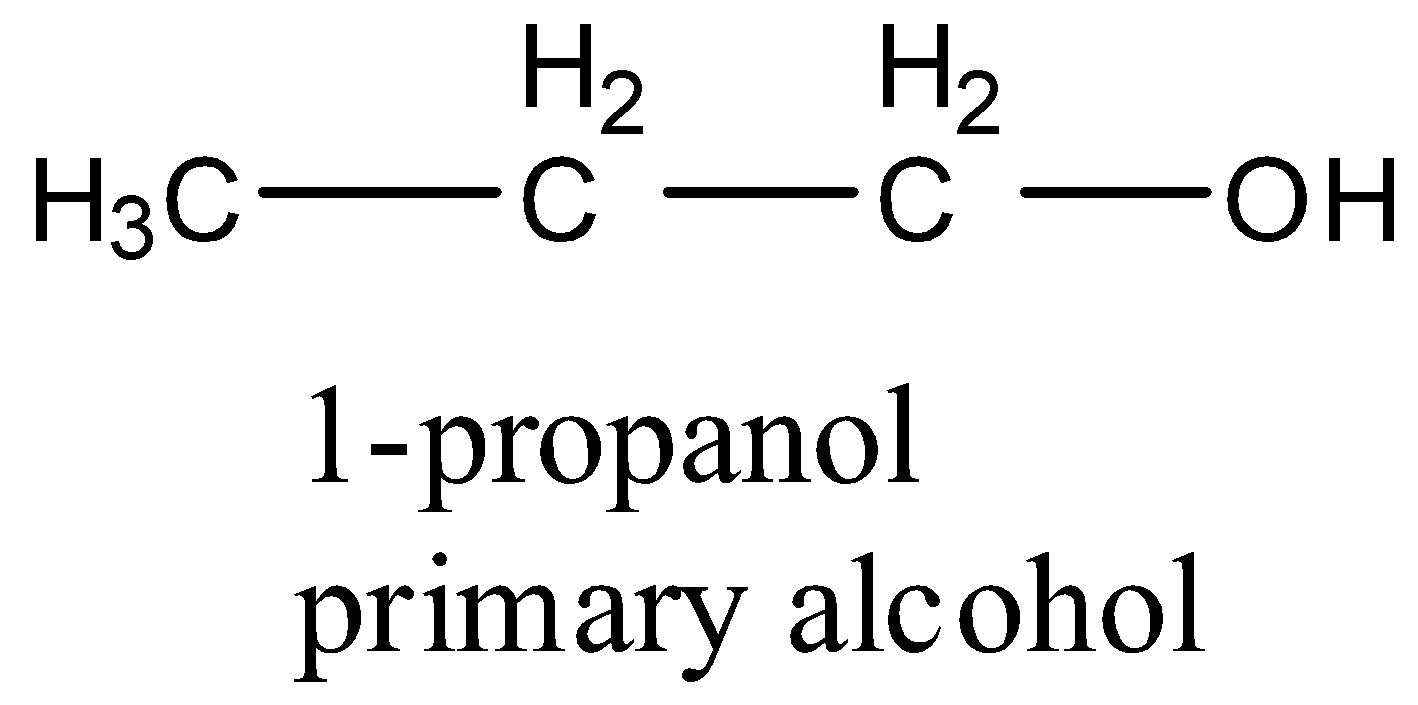
- 1-propanol is an example for primary alcohol.
- Coming to the option C, 1-butanol.
- The structure of 1-butanol is as follows.
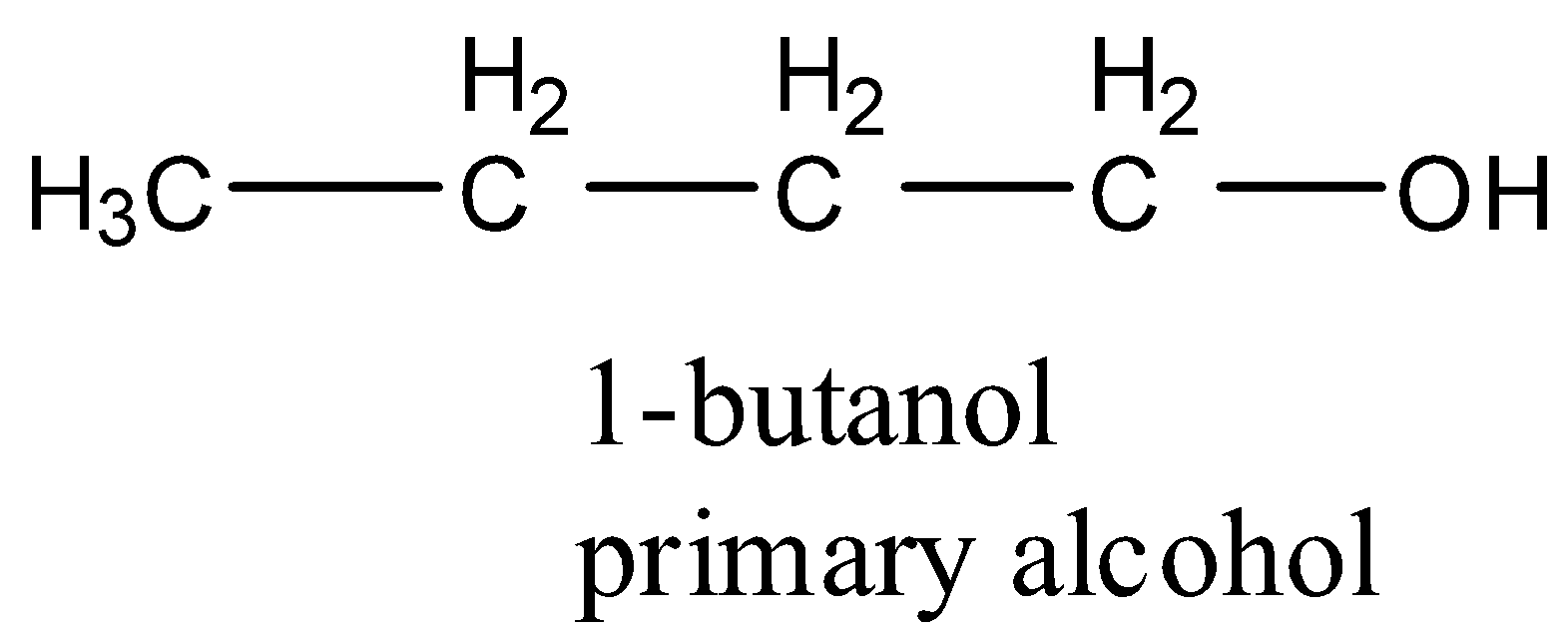
- 1-butanol is a primary alcohol.
- Coming to option D, 2-pentanol.
- The structure of 2-pentanol is as follows.
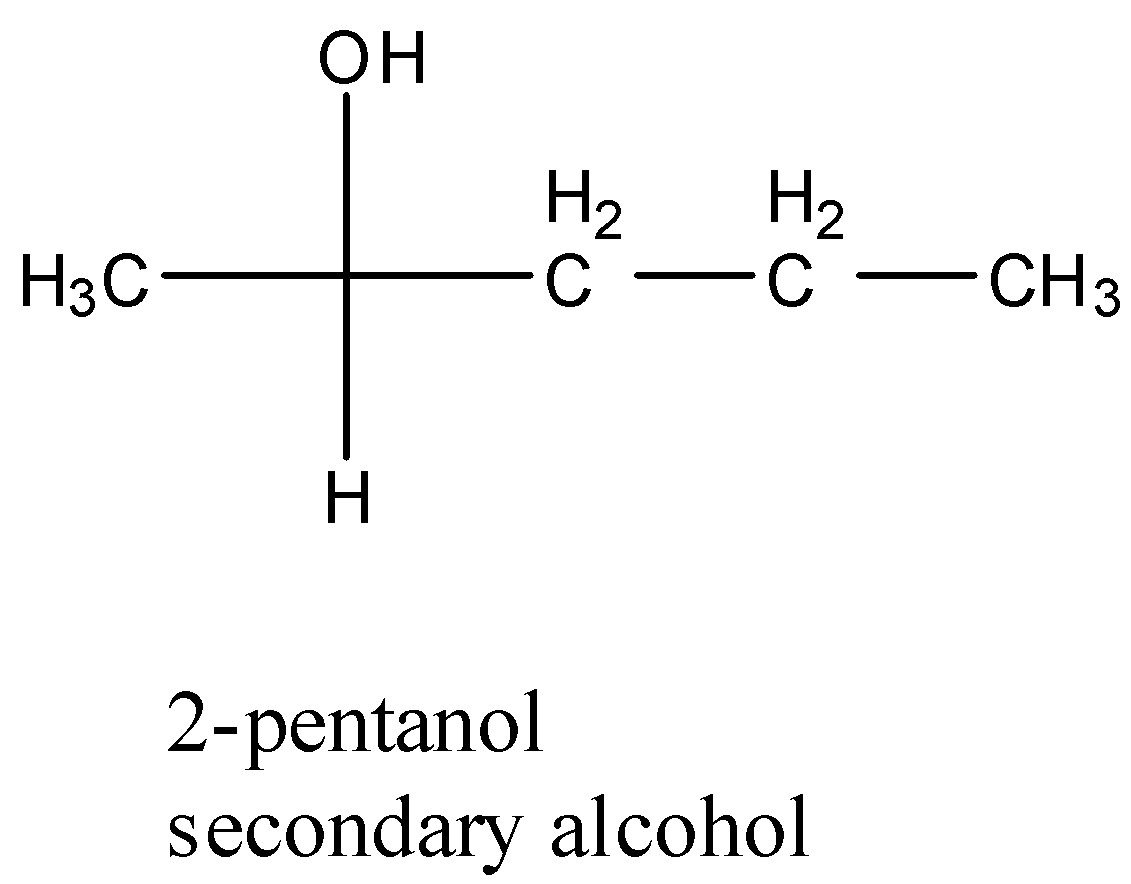
- The compound 2-pentanol is a secondary alcohol.
Therefore the correct option is D.
Note:
Primary alcohol is more reactive than the secondary and tertiary. The reason behind the less reactivity of the tertiary alcohols is due to an increase of +I effect or increased number of alkyl groups in the organic compound.
