Question
Question: Which of the following is a secondary alcohol? (A)  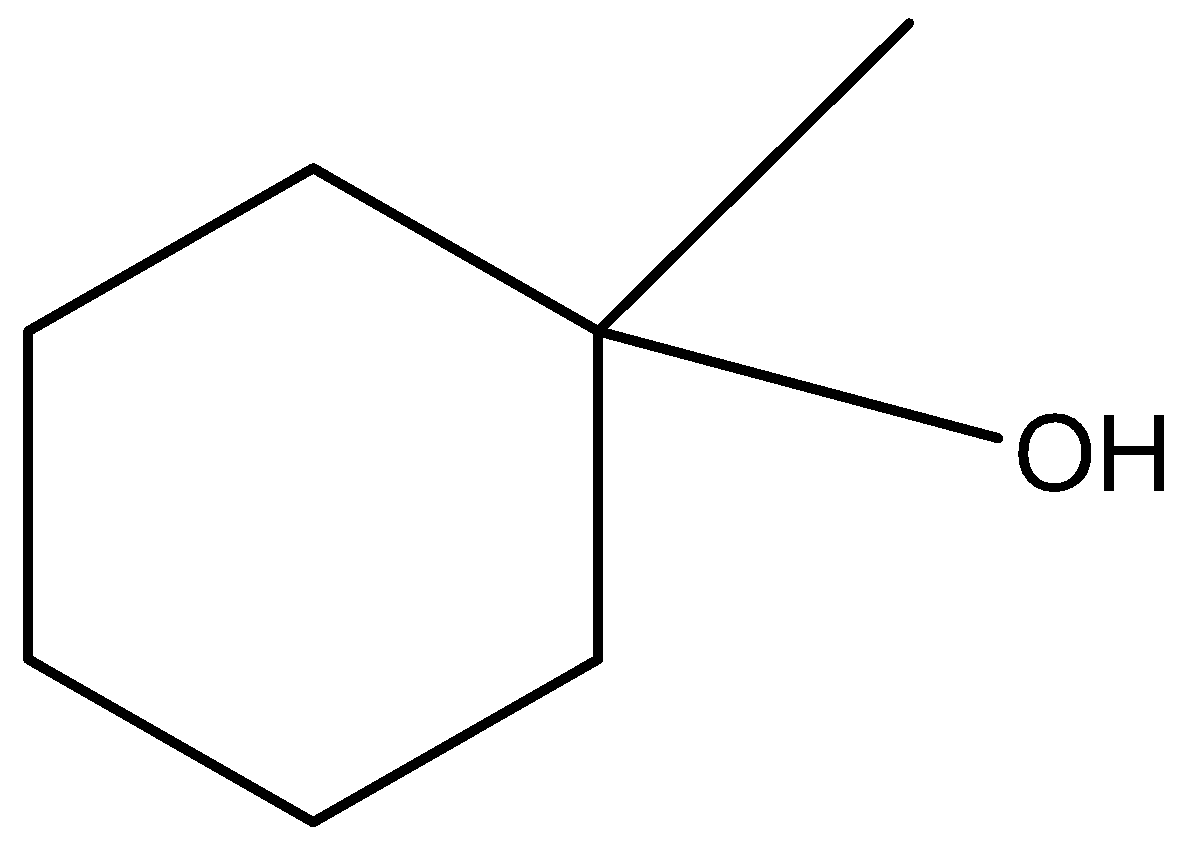
(B) 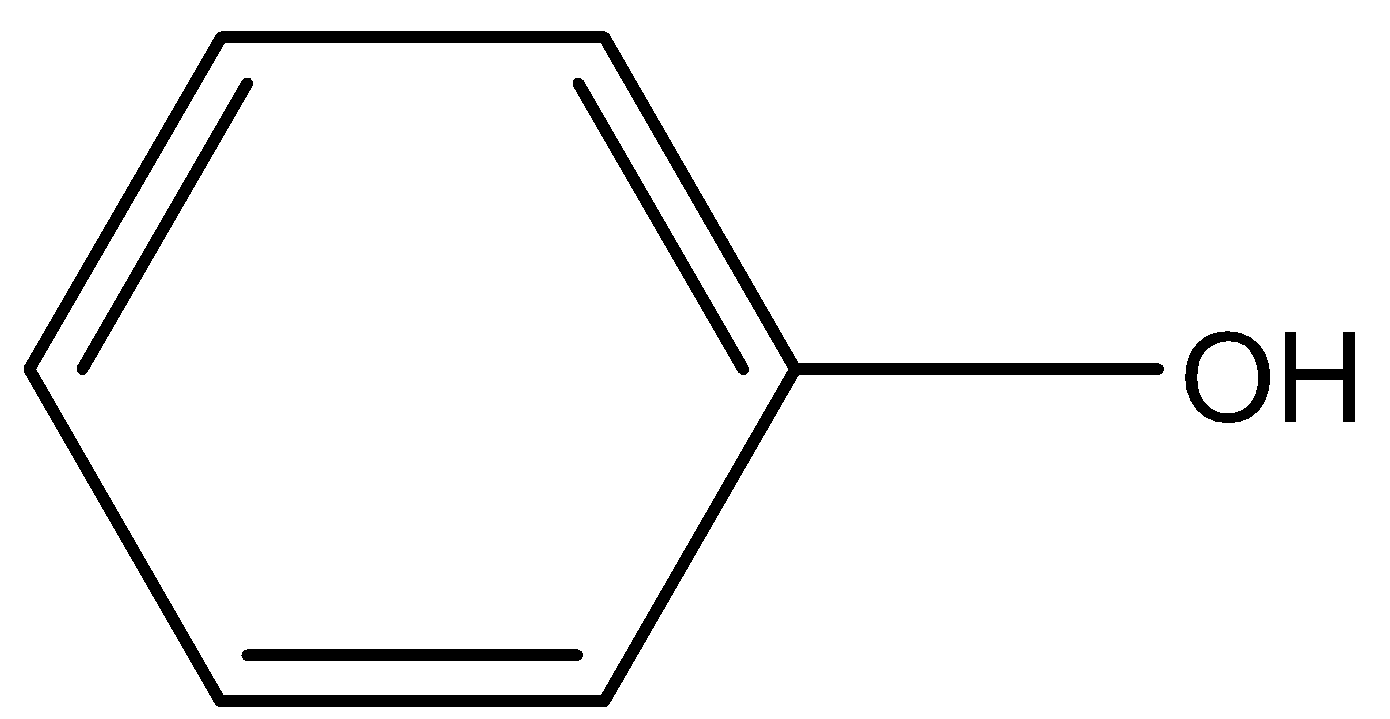
(C) 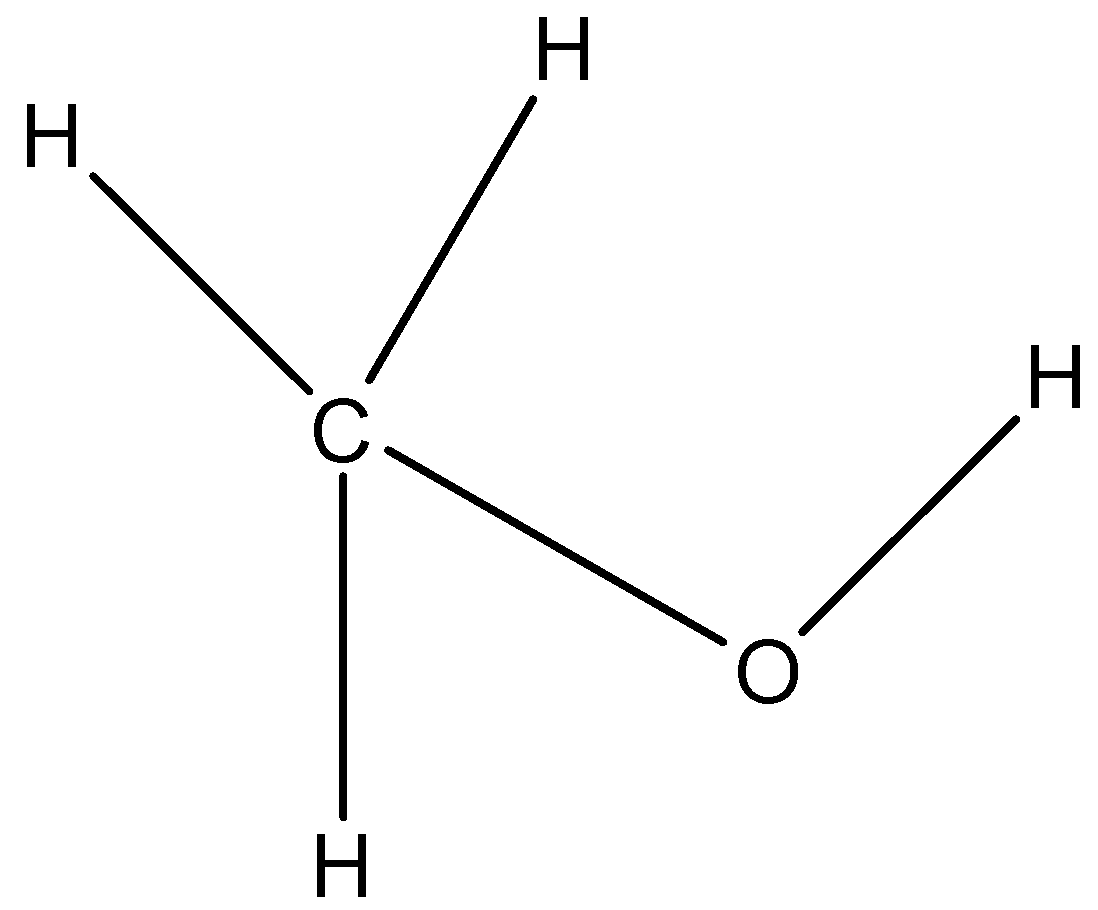
(D) 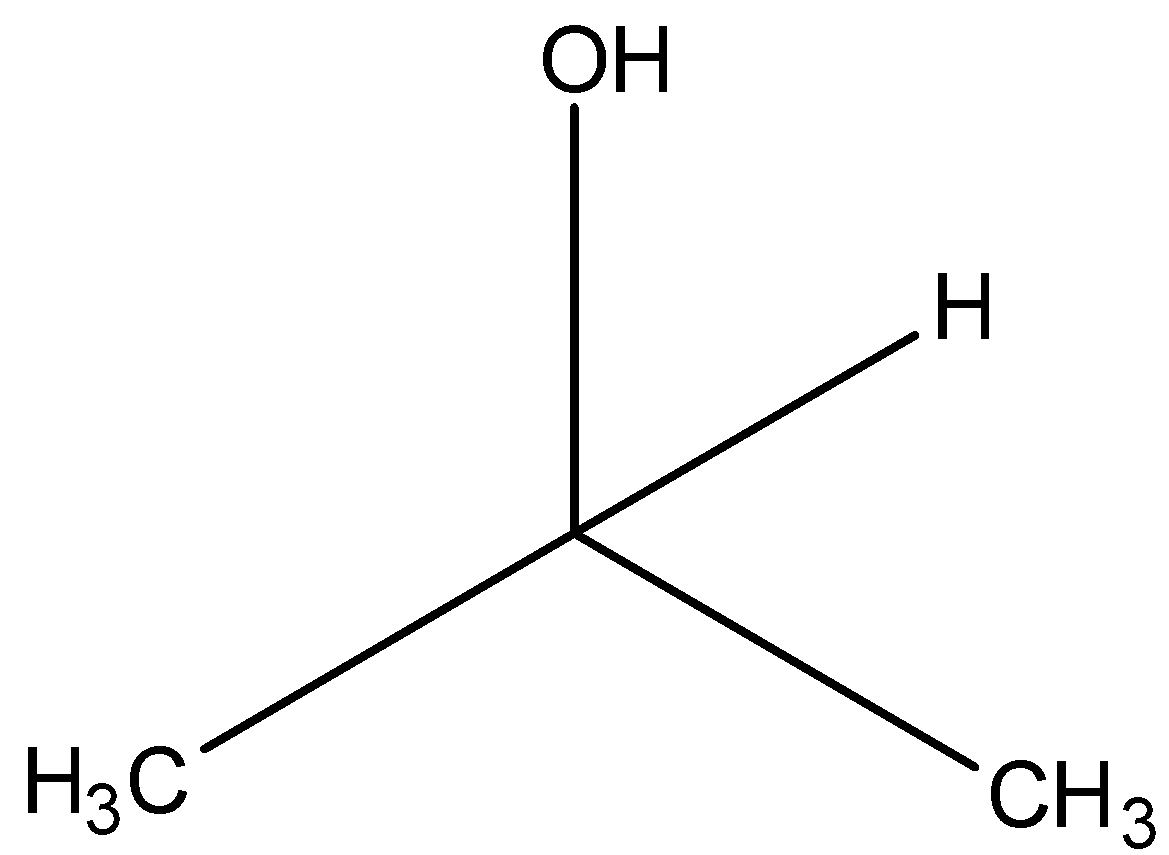
Solution
Hint The carbon attached to two other carbon atoms or groups is called a secondary carbon. If a hydroxyl group is attached to this carbon, the compound becomes a secondary alcohol. So, find the carbon atom which is attached to other carbon groups or atoms and has a hydroxyl functional group.
Complete step-by-step answer:
As suggested in the hint above, we will find the compounds having a secondary carbon having a hydroxyl group attached to it.
(a)- 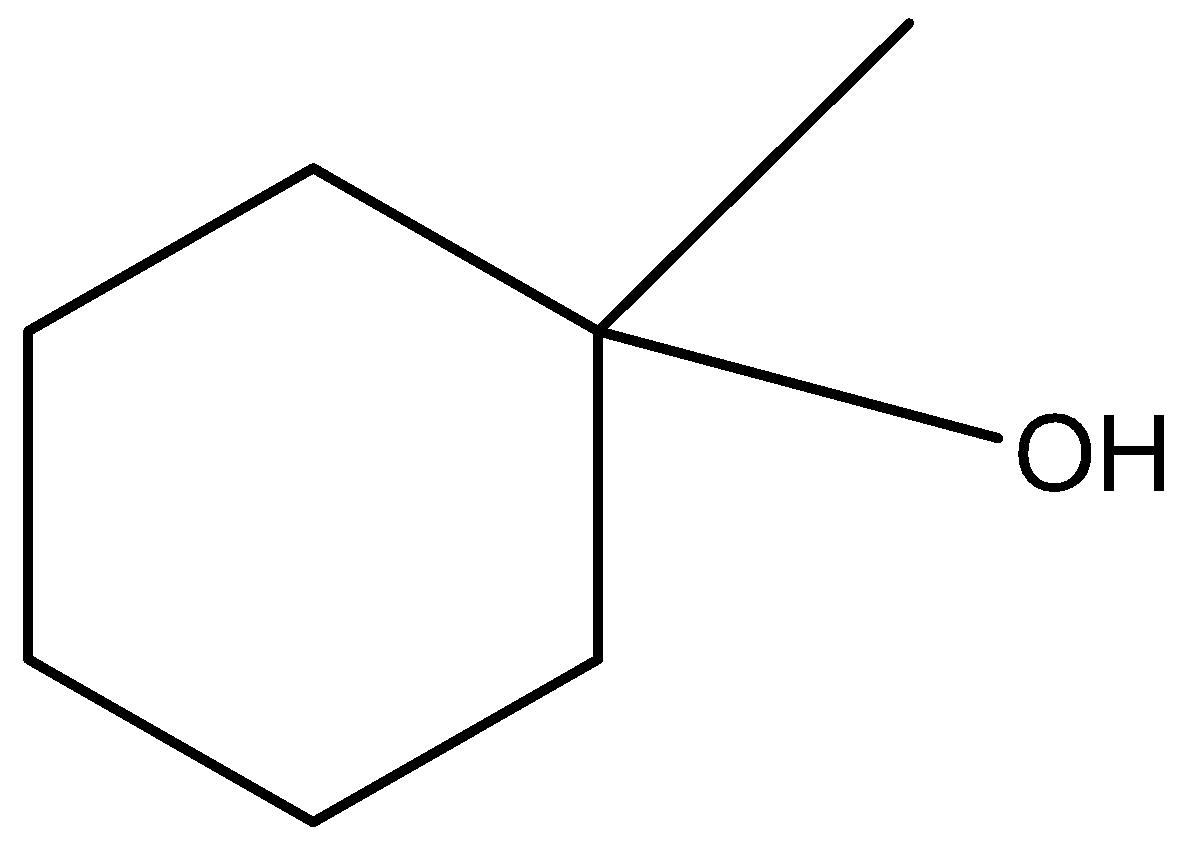
The carbon atom having the hydroxyl functional group (-OH) has 3 carbon atoms attached to it. Hence it is not a secondary alcohol, instead it is a tertiary alcohol.
(b)- 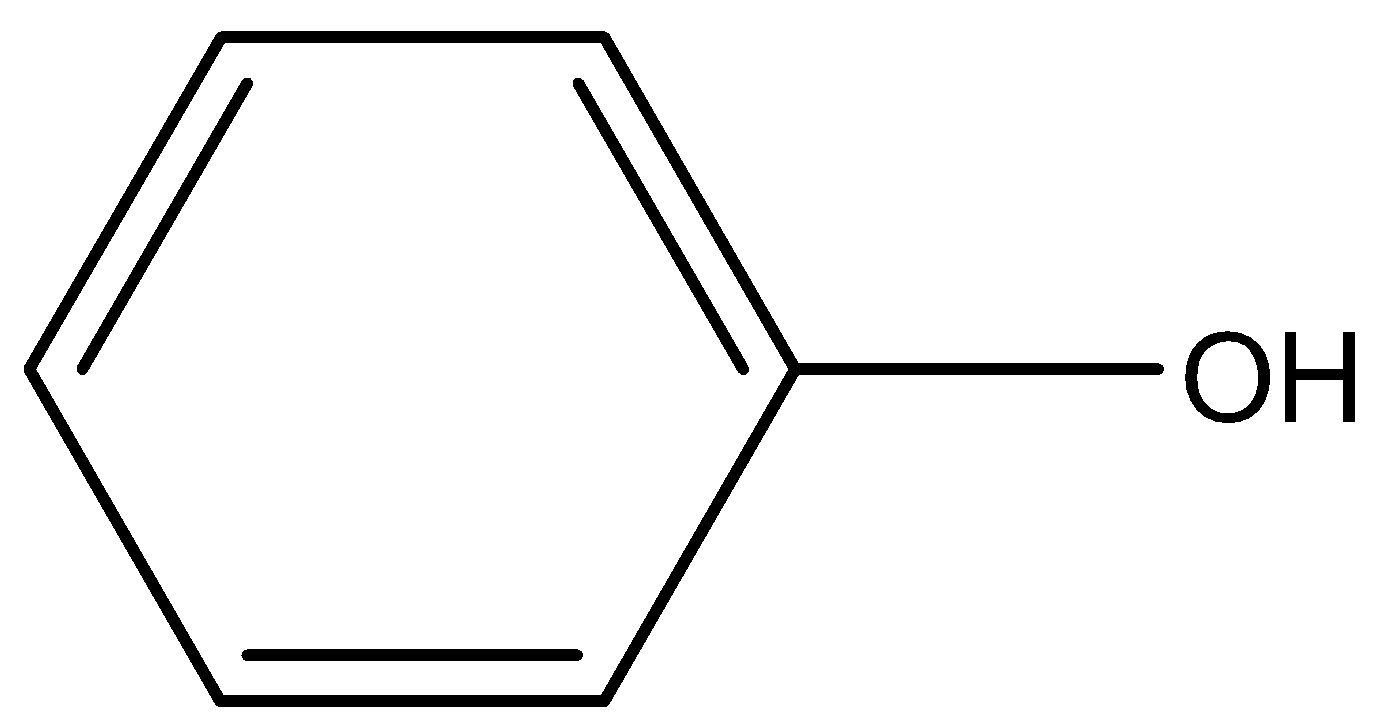
The carbon atom having the hydroxyl functional group (-OH) has 2 carbon atoms attached to it. Hence, it is a secondary alcohol. Option (B) is one of the correct answers.
(c)- 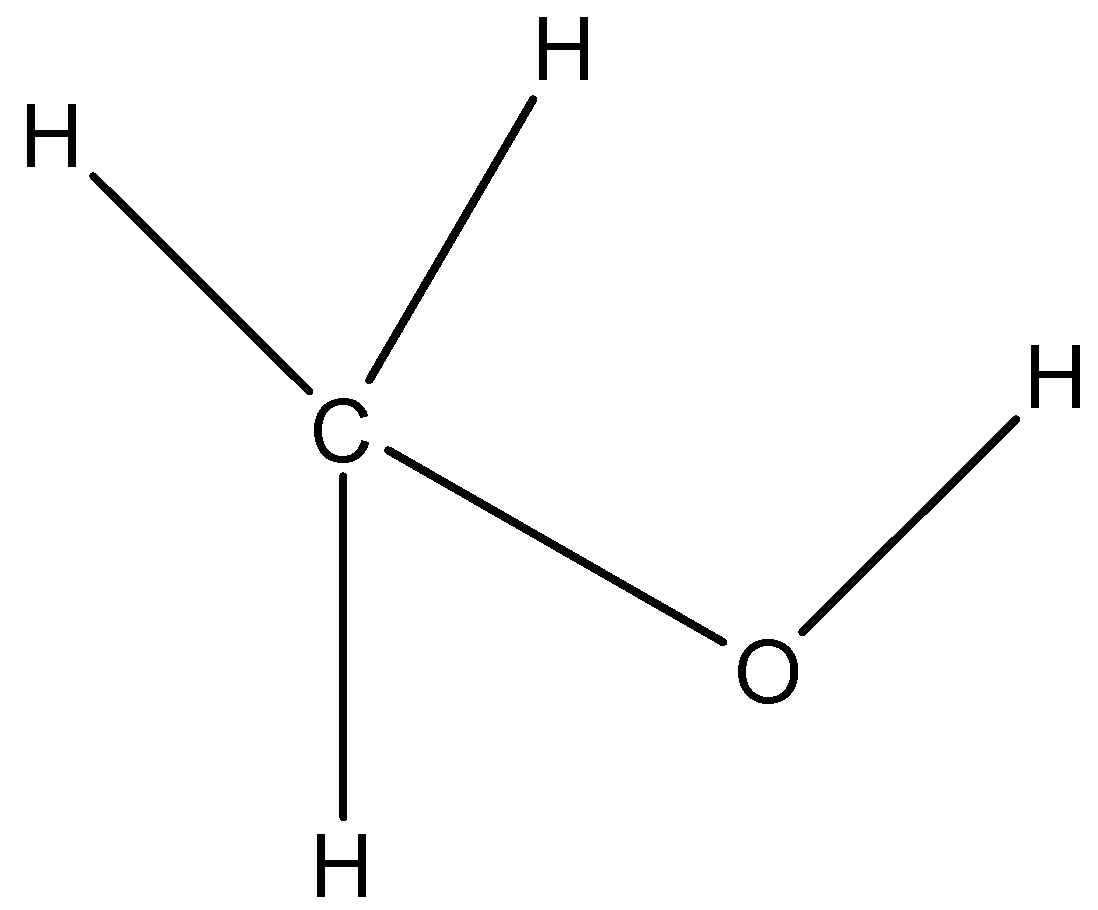
The carbon atoms having the hydroxyl functional group (-OH) is not connected to any carbon atom. Hence it is a primary alcohol and not a secondary alcohol.
(d)- 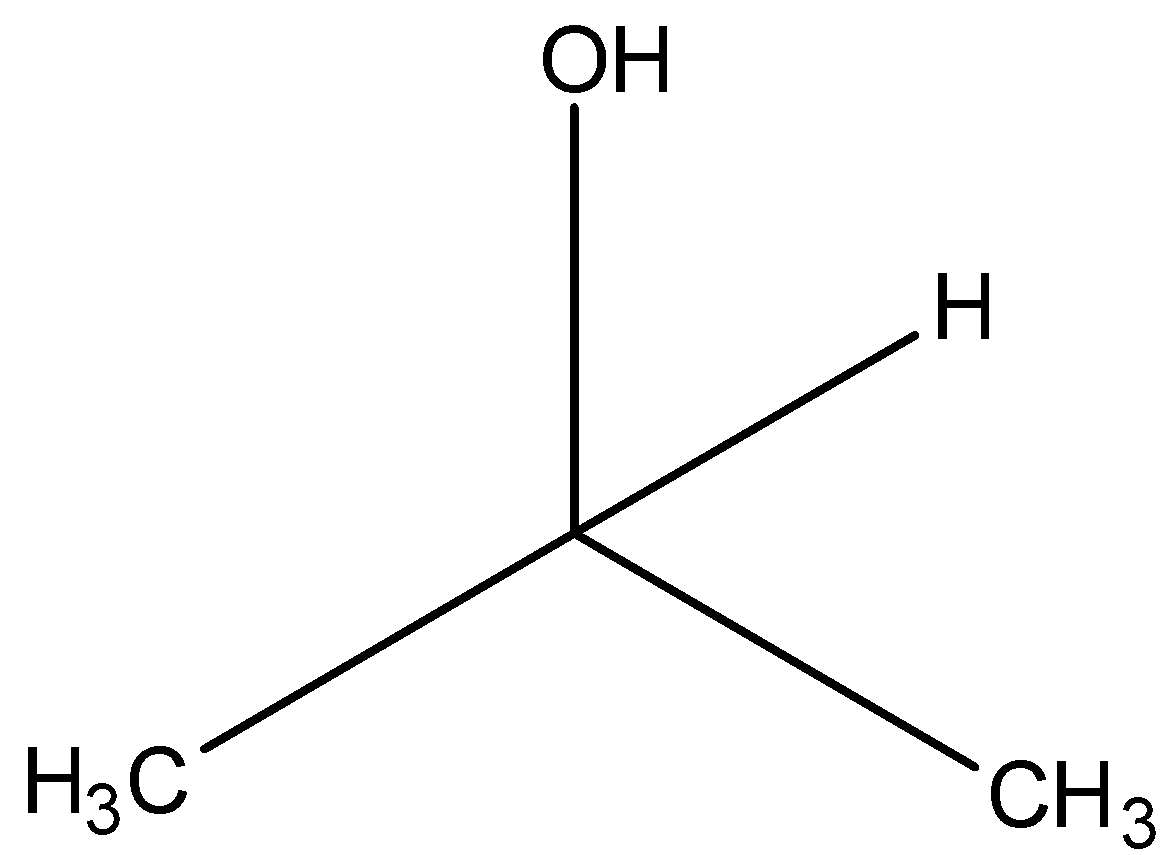
The carbon atom having the hydroxyl functional group (-OH) has 2 carbon atoms attached to it. Hence it is a secondary alcohol. Option (B) is one of the correct answers.
Therefore, the correct answers are options (B) and (D).
Note: Primary, secondary and tertiary alcohols can be separated by adding Lucas reagent. Lucas reagent consists of Zinc in the presence of HCl. Tertiary alcohols react the fastest and form turbidity in the solution. On the other hand, primary alcohols react the slowest and form turbidity after long standing.
