Question
Question: Which of the following is a ring chain of i-butene? 
C. Both A and B
D. none of these
Solution
The molecules having the same molecular formula but a different arrangement of atoms are known as isomers. We will determine the molecular formula of each given compound to determine whether it is an isomer of i-butene or not. To determine the molecular formula we will count the number of each type of atom.
Complete solution:
The structure of i-butene is as follows:
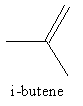
i-butene has four carbon atoms and eight hydrogen atoms. So, the molecular formula of i-butene is C4H8 .
As we knew the isomers have same molecular formula so, we will determine the molecular formula of compound A and B as follows:
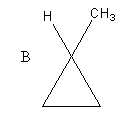
The structure of the given compounds A and B is as follows:
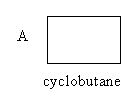
Compound A has four carbon atoms and eight hydrogen atoms. So, the molecular formula of compound A, cyclobutane is C4H8 .
Compound B also has four carbon atoms and eight hydrogen atoms. So, the molecular formula of compound B, is C4H8 .
So, as the molecular formula of compound A and B is the same as of i-butene so, compound A and B are the isomers of i-butene.
So, A and B both are the ring chain of i-butene.
Therefore, the correct answer is (A).
Note: Isomers have the same molecular formula but different chemical formula. A molecular formula shows the total number of an atom in the compound. The chemical formula shows the different groups of atoms of a molecule. To draw the structure of the isomers from the given molecular formula first we determine the degree of unsaturation by using the following formula:
