Question
Question: Which of the following is a red liquid? A. \(S{F_4}\) B. \(S{F_6}\) C. \(SC{l_2}\) D. \({...
Which of the following is a red liquid?
A. SF4
B. SF6
C. SCl2
D. S2Cl2
Solution
A chemical gets its color by electrons that absorb energy and become excited. The colour basically comes from the excitation of electrons due to the absorption of energy performed by the chemical. Red liquid generally refers to a solution that has a red color and is liquid at room temperature.
Complete step by step answer:
Basically a red liquid is the one which has a reddish- brown appearance and is liquid at room temperature.
Among the given options SCl2 (Sulphur dichloride) is considered as a red liquid because it is red in colour and is liquid at room temperature. This cherry-red liquid is the simplest sulfur chloride and is most commonly used.
The structure of the compound is as shown:
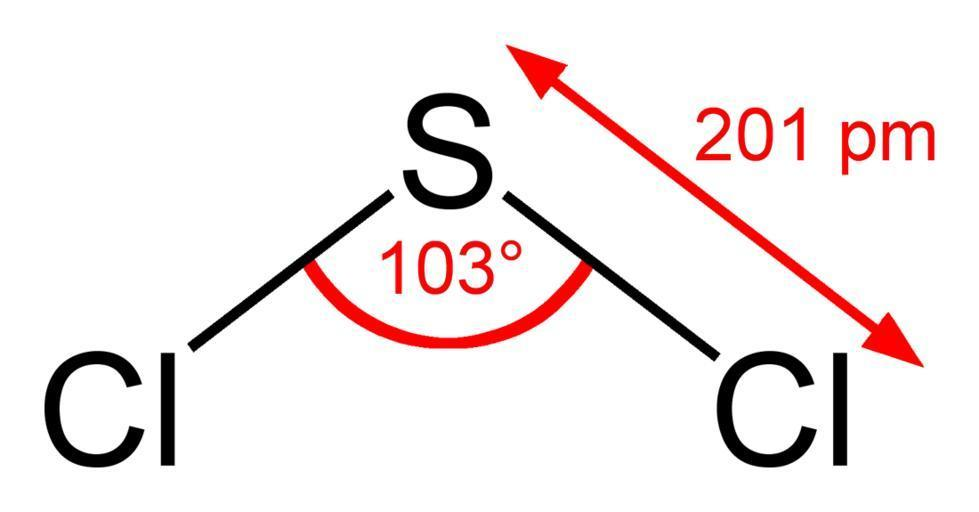
As shown in the figure, it seems to have a bent shape with asymmetric charge distribution. It further contains one Sulphur and two chlorine molecules. It has a bond angle of 103∘ and bond length of 201pm
\
Hence, option C is correct.
Note: SF4 I.e. Sulphur tetrafluoride is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture, SF6 i.e. sulfur hexafluoride is also colorless,odorless, non-flammable and non-toxic gas and S2Cl2 i.e. disulphur dichloride is a light-amber to yellow oily liquid.
