Question
Question: Which of the following is a non-reducing sugar? A. Glucose B. Sucrose C. Maltose D. Lactose...
Which of the following is a non-reducing sugar?
A. Glucose
B. Sucrose
C. Maltose
D. Lactose
Solution
Non-reducing sugars does have a group attached to any of the anomeric carbon. Therefore, they are unable to reduce other compounds.
Complete step by step answer:
We can draw the structure of sucrose as follows:
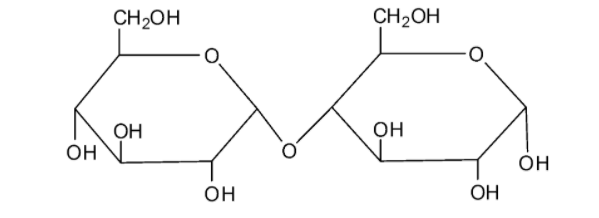
The molecule of sucrose is a disaccharide. From the structure, we can see that sucrose is a combination of the monosaccharides fructose and glucose with the formula C12H22O11. It is a non-reducing sugar as this molecule does not have characteristics of the reducing sugars. The two monosaccharide units are connected by a glycosidic linkage between C1 of α− glucose and C2 of β− fructose. As the reducing groups of the glucose molecule and fructose molecules are involved in the formation of the glycosidic, sucrose is considered a non-reducing sugar.
Therefore, Sucrose is a non-reducing sugar.
So, out of the given four options, B is the correct option.
Additional information:
We can draw the structure of glucose as follows:
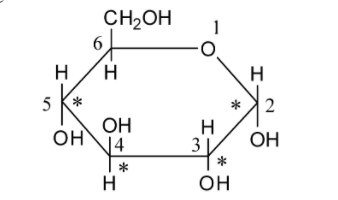
Glucose is a monosaccharide. As glucose acts as a reducing agent, it is reconsidered as a reducing sugar.
We can draw the structure of maltose as follows:
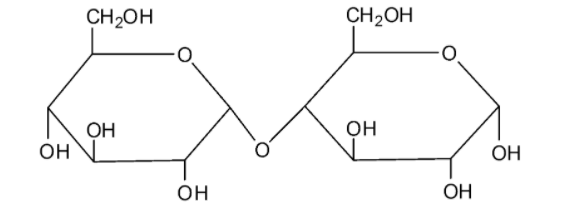
Maltose is made by the combination of two glucose molecules. Maltose undergoes mutarotation. Because of this reason, maltose is considered as a reducing sugar.
We can draw the structure of lactose as follows:
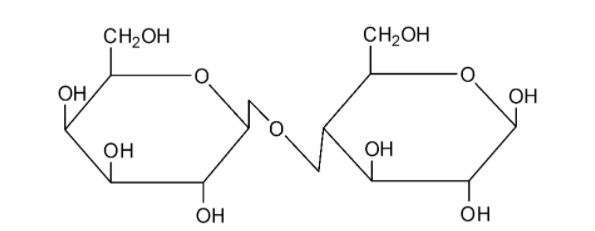
Lactose is composed by the combination of a glucose and a galactose molecule. Lactose undergoes mutarotation and it is hence a reducing sugar.
Note:
Monosaccharides are considered as reducing sugars. Then sugar that cannot act as a reducing agent are non-reducing agents. Out of disaccharides, sucrose is a well-known non-reducing sugar.
