Question
Question: Which of the following is a non-aromatic compound? A. 
B.
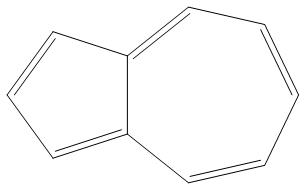
C.
D.
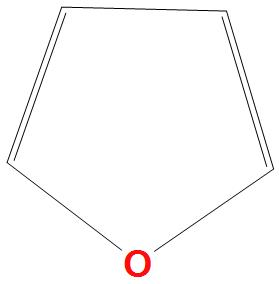
Solution
Aromatic compounds are those that obeys Huckel’s rule, are planar and will show cyclic delocalisation. Huckel’s rule is (4n+2)πe−, where n is the number of benzene rings. For n=1, there should be 6 pi electrons. For n=2, there should be 10 pi electrons and so on.
Complete Solution :
- A. 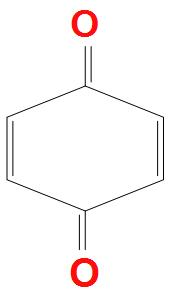
There are carbonyl pi electrons present, but these do not resonate within the ring, that’s why they are not aromatic. Second reason is that there is also no proper conjugation present, and only cross conjugation is present. It doesn’t obey Huckel’s rule for aromaticity. Hence, it is a non-aromatic compound.
- B.

It is having 10 pi electron, and is an aromatic compound as it obeys Huckel’s rule(4n+2)πe−:
