Question
Question: Which of the following is a monomer of Teflon? (a) Difluoroethane (b) Trifluoroethane (c) Tetr...
Which of the following is a monomer of Teflon?
(a) Difluoroethane
(b) Trifluoroethane
(c) Tetrafluoroethane
(d) None of these
Solution
Hint: Teflon is a polymer which is used widely in our daily lives. It is very popularly used as a non-stick coating in cooking utensils.
Complete step by step answer:
The formula of Teflon is (C2F4)n. The structure of Teflon is given below
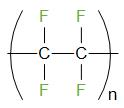
Teflon is also known as PTFE or Polytetrafluoroethene. It is a thermoplastic polymer.
PTFE is manufactured by the addition polymerization of its monomers. It is the reaction which includes free radical polymerization reaction and includes the following steps - initiation, propagation and termination. Therefore, PTFE is an addition polymer.

Tetrafluoroetheneis a monomer of Teflon. The structure of tetrafluoroethene is given below
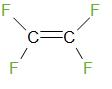
Therefore, the answer is – option (d) – None of these.
Additional Information:
Teflon is the plastic which has the lowest coefficient of friction. As we know, Teflon is very widely used as a non-stick coating for pans and other cookware. Also, being very unreactive Teflon is also very widely used in containers and pipework for reactive chemicals. It is also used as a material to provide resistance to heat & chemical attack. It is also used for making gaskets, pump packing, valves, oil seals, non-lubricated bearings, etc.
Note: The carbons in tetrafluoroethyleneare sp2 hybridized, where two fluorine atoms are bonded to each carbon covalently and the geometry around the carbon atoms is trigonal planar.
