Question
Question: Which of the following is a monomer of Nylon-\(6\)? 
Solution
A monomer is one of the basic units that generally be used to manufacture the polymer unit. The reaction of the formation of this polymer is termed as polymerization. The Nylon−6 is one of the polymers which is a semicrystalline polyamide.
Step by step answer:
The monomers have different names depending upon the number of monomeric molecules like Dimer polymer containing two monomers, Trimer containing three monomers, tetramer containing four monomer molecules, pentamer containing five monomer units, etc. There are various examples of monomeric molecules. Some are-glucose, vinyl chloroethylene are examples of monomers.
Natural rubber may be considered a linear polymer of isoprene (2−methyl−1,3−butadiene) and called cis−1,4−polyisoprene. Here isoprene is the monomer unit of rubber. Similarly, Neoprene or polychloroprene is formed by the free radical polymerization of chloroprene.
Nylon−6 is a Polyamides. These polymers must have an amide linkage. The general method of preparation consists of the condensation polymerization of diamines with dicarboxylic acids and also of amino acids and their lactams.
It is obtained by heating caprolactam whose structure is shown below with water at a high temperature.
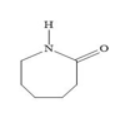
Nylon−6 fibers are somewhat strong and have high tensile strength. These have a special property of resistivity to the acids. These are used in apparel, hosiery, seat belts, and parachutes ropes.
Hence, option (B) is correct.
Note: The condensation polymerization of hexamethylenediamine prepares nylon 6,6 with adipic acid under high pressure and at high temperatures. Nylon 6,6, is used in making sheets, bristles for brushes, and in the textile industry.
