Question
Question: Which of the following is a monobasic acid? A) \[{H_2}S{O_4}\] B) \[C{H_3}COOH\] C) \[{H_3}P{O...
Which of the following is a monobasic acid?
A) H2SO4
B) CH3COOH
C) H3PO3
D) H2CO3
Solution
The basicity of acid is defined as the number of replaceable hydrogen present in an acid molecule. So, by the ionization of all the given acid molecules, the one which does not replace more than one hydrogen will be the monobasic acid.
Complete step by step answer:
Let us first see the structure of H2SO4.
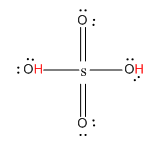
Here, the hydrogen marked with red color is the transferrable hydrogen atom. As there are two transferable hydrogen atoms, H2SO4 is the dibasic acid.
Now, let us see the structure of H3PO3.
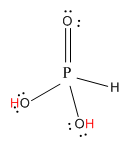
Here, the hydrogen marked with red color is the transferrable hydrogen atom. As there are two transferable hydrogen atoms, H3PO3 is the dibasic acid.
Now, let us see the structure of H2CO3.
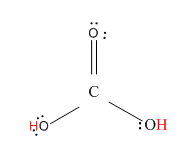
Here, the hydrogen marked with red color is the transferrable hydrogen atom. As there are two transferable hydrogen atoms, H2CO3 is the dibasic acid.
Now, let us see the structure of CH3COOH.
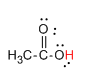
Here, the hydrogen marked with red color is the transferrable hydrogen atom. As there are only one transferable hydrogen atom, CH3COOH is the monobasic acid.
We can also show the ionizable hydrogen atom by dissociation of the acetic acid molecule as follows.
CH3COOH→CH3COO−+H+
The above reaction shows only one ionizable hydrogen atom. So, its basicity is also one.
Therefore, we can conclude that the correct answer to this question is option B.
Note:
The hydrogen attached to the oxygen is only replaced and the hydrogen attached to the central atom is irreplaceable as in phosphorous acid and acetic acid. Always focus on the structure for this type of question.
