Question
Question: Which of the following is a good conductor of electricity? A.Charcoal B.Coke C.Graphite D.Di...
Which of the following is a good conductor of electricity?
A.Charcoal
B.Coke
C.Graphite
D.Diamond
Solution
Conductors are defined as the materials that allow the electricity to flow through them easily. Further, this property of conductors that allow them to conduct electricity is known as conductivity.
Complete step by step answer:
We usually differentiate the elements around us based on their physical properties such as texture, polarity, solubility, color etc. But another very important classification of elements is done on the basis of their conductivity of electric charges i.e. conductors and insulators. Now, conductors are the materials that allow electricity to flow through them easily. This property of conducting electricity is known as conductivity. Moreover, the flow of electrons in a conductor is known as electric current and the force required to make that current flow through the conductor is known as voltage.
Now, from the given options graphite is the good conductor of electricity. It is a type of carbon crystal and is considered as one of the allotropes of carbon. This crystal carbon has a structure that is planar and layered. Further, there is a covalent bonding for atoms on the plane with the criteria being met by only three out of four probable bonding sites. Moreover, the layers of the carbon crystal could swiftly move past each other as the layers could be separated easily as weak van der Waals forces hold them together. The structure is as shown:
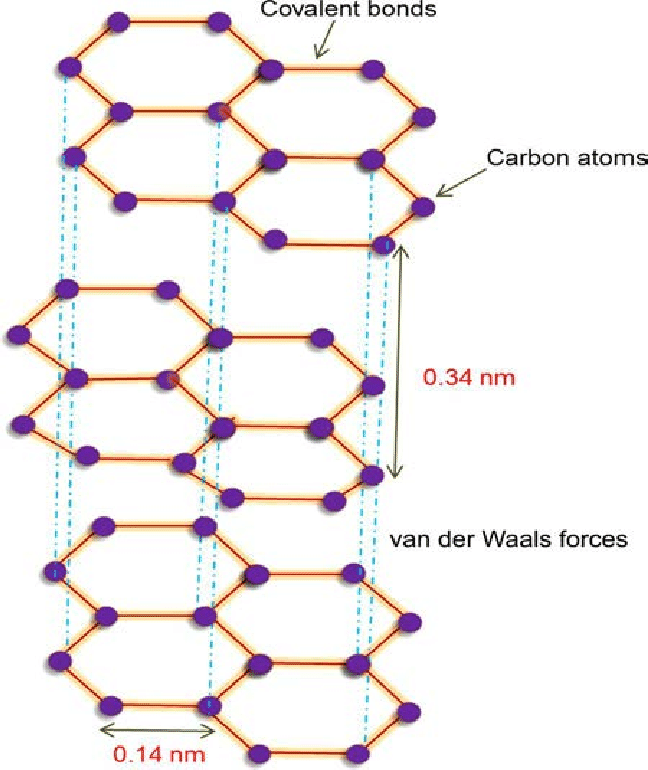
Hence, option C is correct.
Note: In modern times, Graphite is usually consumed in steelmaking, lubricants, batteries etc. Moreover, one of the most important components of graphite i.e. graphene has special features and is one of the widely known strong materials.
