Question
Question: Which of the following is a diatomic gas? (A) Hydrogen (B) Oxygen (C) Chlorine (D) All of th...
Which of the following is a diatomic gas?
(A) Hydrogen
(B) Oxygen
(C) Chlorine
(D) All of the above
Solution
In a free state and at standard temperature and pressure, some elements exist as diatomic molecules to achieve stability. In these diatomic molecules, both the atoms are of the same element and hence are homonuclear.
Complete answer:
To determine whether the gas exists as a diatomic molecule, we need to check the stability of the diatomic molecule formed.
(A) Hydrogen atom has 1 electron in its valence shell and needs 1 more electron to form a complete outermost shell. So when two hydrogen atoms form a bond, both the hydrogen atoms share two electrons between them, forming a covalent single bond and completing the octet.
Its Lewis structure is as follows:
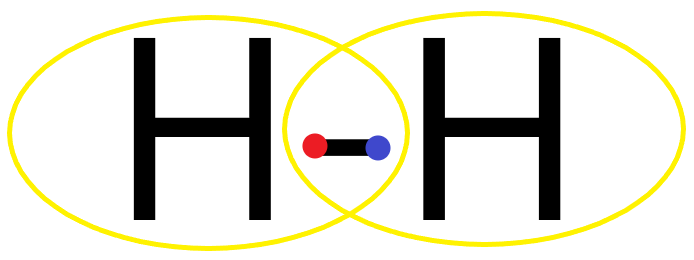
Hence H2 is stable and exists as a diatomic gas.
(B) Oxygen atom has 6 electrons in its valence shell and needs 2 more electrons to form a complete outermost octet shell. So, when 2 oxygen atoms form a bond, both the oxygen atoms share 4 electrons between them, forming a covalent double bond and completing the octet.
Its Lewis structure is as follows:
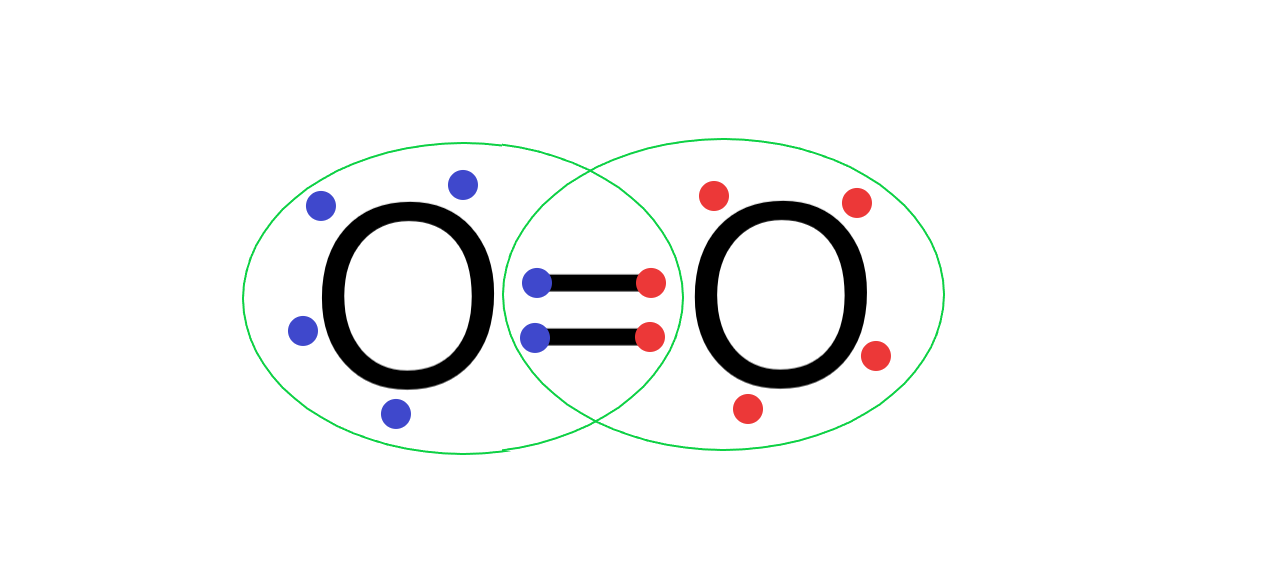
Hence O2 is stable and exists as a diatomic gas.
(C) Chlorine atom has 7 electrons in its valence shell and needs 1 more electron to form a complete outermost octet shell. So when 2 chlorine atoms form a bond, both the chlorine atoms share 2 electrons between them, forming a covalent single bond and completing the octet.
Its Lewis structure is as follows:
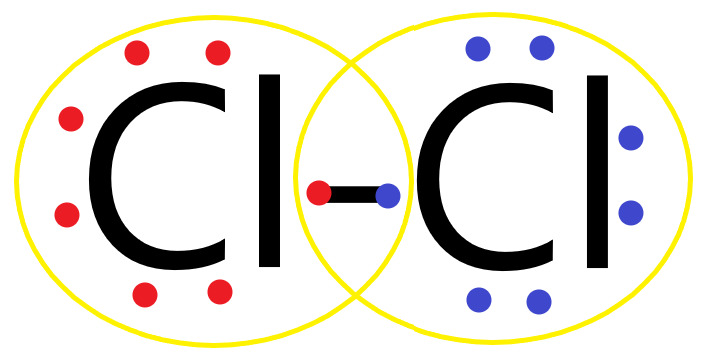
Hence Cl2 is stable and exists as a diatomic gas.
So, the correct answer is option (D) All of the above.
Note:
It should be noted that other than chlorine Cl2, oxygen O2, and hydrogen H2, the only other gases that exist as homonuclear diatomic molecules at STP are fluorine F2 and nitrogen N2.
At temperatures higher than STP, bromine Br2 , and iodine I2 also exist as diatomic molecules.
