Question
Question: Which of the following is a diamagnetic ion? (A) \[Z{n^{2 + }}\] (B) \(N{i^{2 + }}\) (C) \(C...
Which of the following is a diamagnetic ion?
(A) Zn2+
(B) Ni2+
(C) Co2+
(D) Cu2+
Solution
There are two types of atoms. Paramagnetic and diamagnetic. When two electrons are paired in an orbital such that their total spin is equal to zero, then it is known as a diamagnetic atom. In other cases, if electrons are unpaired then the atom is called as paramagnetic.
Complete step by step answer: If we consider all the given options, we can find out whether the ions are paramagnetic or diamagnetic by knowing their electronic configuration.
(i) Zn2+
Atomic number of Zinc = 30
∴ Numbers of electrons in Zn+=30−2⇒28 electrons
Electronic configuration of Zn+
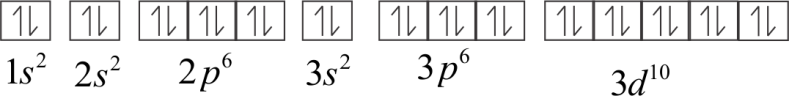
Or 1s22s22p63s23p63d10
All the electrons in Zn2+ are paired. Therefore, the total spin value is zero and it is a diamagnetic ion.
(ii) Ni2+
Atomic number of Ni=28
∴ Number of electrons in Ni2+=28−2⇒26electrons
Electronic configuration of Ni2+
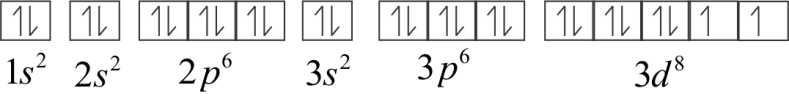
Or 1s22s22p63s23p63d8
In 3d orbital, two electrons are unpaired. Therefore, Ni2+ is paramagnetic.
(iii) Co2+
Atomic number of Co=27
Number of electrons in Co2+=27−2⇒25 electrons
Electronic configuration of Co2+
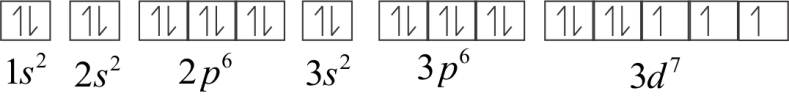
Or 1s22s22p63s23p63d7
In 3d orbital, three electrons are unpaired. Therefore Co2+ is paramagnetic.
(iv) Cu2+
Atomic number of Cu=29
Number of electrons in Cu2+=29−2⇒27 electrons
Electronic configuration of Cu2+
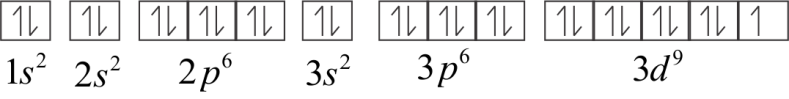
Or 1s22s22p63s23p63d9
In 3d orbital, one electron is unpaired. Therefore, Cu2+ is paramagnetic.
Hence, among all the options, only Zn2+ is diamagnetic ion.
Note: Even if only one unpaired electron is present in an atom and rest all electrons are paired. Still, the atom would be considered as paramagnetic as its total spin would not be equal to zero.
