Question
Question: Which of the following is a cyclic oxoacid? (A) \( {H_4}{P_2}{O_7} \) (B) \( {H_4}{P_2}{O_6} \)...
Which of the following is a cyclic oxoacid?
(A) H4P2O7
(B) H4P2O6
(C) H3P3O9
(D) H5P5O15
Solution
Hint : Oxoacids (also known as oxyacids) are acids that contain oxygen. An oxoacid, to be more specific, is an acid that contains oxygen and contains at least one additional component that has one or more hydrogen atoms bound to oxygen. And cyclic oxoacids is an oxoacid whose chemical structure is cyclic.
Complete Step By Step Answer:
Now, to analyse the structure of the compounds first we need to make the chemical structures of all the compounds in the option.
Option (A) H4P2O7
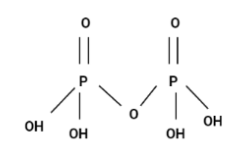
Since, the structure is a chain and it's not cyclic. Hence, option (A) is incorrect.
Option (B) H4P2O6
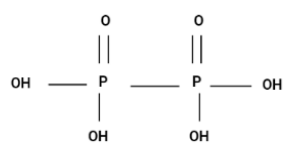
Since, the structure is a chain and it's not cyclic. Hence, option (B) is also incorrect.
Option (C) H3P3O9

This structure is cyclic oxoacid. Hence, the correct option is (C).
Option (D) H5P5O15

The structure is a chain and it’s not cyclic. This structure is the same as option (C) , the only difference is that this structure is chain instead of cyclic. Hence, option (D) is also incorrect.
Therefore the correct option is (C).
Note :
Oxoacids are acids that contain oxygen as a component. Phosphorus is known to shape a variety of oxoacids, including H3PO4 , H3PO3 , and others. It is tetrahedrally surrounded by other atoms in phosphorus oxoacids.
