Question
Question: Which of the following is a co-polymer? A.Polytetrafluoroethylene B.Polyvinyl chloride C.Polye...
Which of the following is a co-polymer?
A.Polytetrafluoroethylene
B.Polyvinyl chloride
C.Polyethylene
D.Nylon- 6, 6
E.Natural rubber
Solution
A polymer made by reaction of more than one species of monomer is known as copolymer and the process of polymerization of two or more different monomers into copolymers is called copolymerization.
Complete step by step answer:
Polytetrafluoroethylene is also commonly called as Teflon. This polymer is polymerised from its monomer named tetrafluoroethylene by a free- radical polymerization mechanism.
The reaction can be written as:
ηF2C=CF2→(−F2C−CF2−)n
Since, this polymer is made only from one monomer thus it is not a copolymer.
Polyvinyl chloride (PVC) is also known as Polychloroethene. This polymer is made from only one monomer that is vinyl chloride or chloroethylene.
η H2C=CClH→(−H2C−CClH−)n
This polymer is also not a copolymer since it is polymerised from one monomer only.
Polyethylene or polythene (PE) is also called polymethylene. The monomer used for the polymerization process of this polymer is ethylene.
ηH2C=CH2→(−H2C−CH2−)n
Therefore, it is also not a copolymer.
-Nylon- 6, 6 is a polymerised from two different monomers that are hexamethylenediamine and adipic acid by the process known as Polycondensation. The reaction can be shown as:
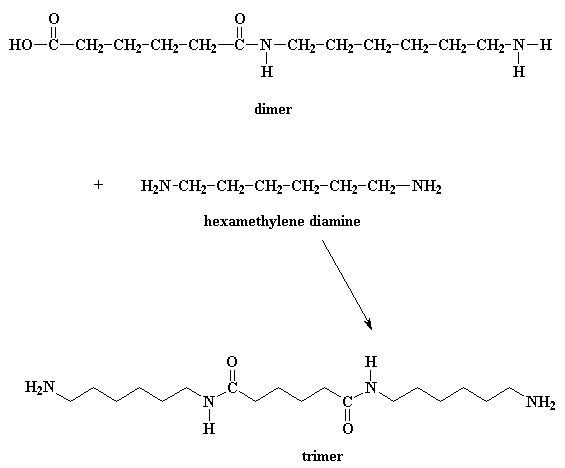
Since, this polymer is polymerised from two different monomers, hence it is a copolymer.
-Natural rubber is a polymer of isoprene.i.e. Isoprene is the monomer of natural rubber and natural rubber is also known as Poly-isoprene.
The common name of isoprene is:
2 methyl 1, 3 butadiene
The reaction can be shown as: 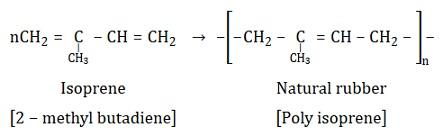
Thus, natural rubber is also not a copolymer.
Hence, the answer is option (D) i.e. Nylon 6,6 is a copolymer.
Note:
One should keep in mind that frequency of light is independent of the medium of the surrounding while wavelength is dependent on the medium of the surrounding. Frequency does not change because it is a property of light while wavelength changes according to the medium in which light is travelling.
