Question
Question: Which of the following hybrids is electron-precise hydride? A. \({{\text{B}}_{\text{2}}}{{\text{H}...
Which of the following hybrids is electron-precise hydride?
A. B2H6
B. NH3
C. H2O
D. CH4
Solution
To answer this question we should know what electron-precise hydride means. The hydrides mean the compound will have hydrogen atoms in the form of an anion. Now come to the electron-precise, it means that an atom that is forming bonds with hydrogen atoms should have the exact number of electrons as it requires to form the normal covalent bonds.
Complete answer:
Electron-precise hybrids contain the exact number of electrons to form a normal covalent bond means the electron number in its valence shell will be equal to the number of covalent bonds formed by it.
We will draw the geometry of each given molecule to determine the electron-precise hydride. The hydride which will have valence electrons equal to the number of the covalent bond will be the electron-precise hydride.
Let us discuss the structure of molecules one by one.
B2H6 is a dimer of BH3 which is generally known as diborane. The valence electronic configuration of boron is 2s22p1. The valence shell of the boron has only three electrons. It requires five electrons to complete its octet. Boron cannot form five bonds, so it BH3forms a dimer to complete its octet.
The structure of the dimer is as follows:
Diborane is electron deficient because each boron has a total of six electrons in four bonds. In this molecule, each boron atom is bonded to 2 terminal hydrogen atoms each also, two boron atoms are held together by two hydrogen atoms in bridging as shown below:
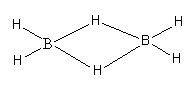
So, as each boron has three valence electrons and forms four covalent bonds, so diborane is electron-precise hydride.
NH3, The valence electronic configuration of nitrogen is 2s22p3. N – atom has 5 valence electrons, out of which 3 electrons form covalent bonds with H-atoms and two electrons remain unbonded. These electrons are called lone pairs of electrons.
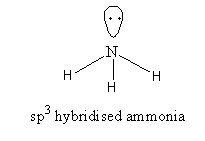
It has a trigonal pyramidal structure. The shape is predicted by valence-shell pair repulsion theory [VSEPR]. The bond angle is 106.7o.
o, the nitrogen has five valence electrons but it forms three covalent bonds. All electrons are not used for bond formation. It has an electron-rich center therefore NH3is not an electron-precise hydride.
H2O, The valence electronic configuration of oxygen is 2s22p4. Oxygen atom has 6 valence electrons of which two electrons form a bond with two hydrogen atoms but 4 electrons [two pairs] remain unbonded.
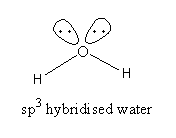
Water has sp3 hybridization but due to the presence of two lone pair of electrons H−O−H angle is 104.5o .
So, the oxygen has six valence electrons but it forms two covalent bonds. All electrons are not used for bond formation. It has an electron-rich centre therefore water is not an electron-precise hydride.
CH4, The valence electronic configuration of carbon is 2s22p2. Carbon atoms have 4 valence electrons. It forms four bonds with four hydrogen atoms.
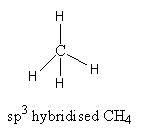
It has tetrahedral geometry with bond angle 109.28o. So, the carbon has four valence electrons and forms four covalent bonds. All electrons are used for bond formation. Therefore, CH4 is electron-precise hydride.
Therefore, option (D) CH4 is the correct answer.
Note: Molecules are either electron-deficient or electron-rich or neutral. If all valence electrons are used to form a covalent bond, then the molecule is precise. The electron-precise molecules have perfect or we can say regular geometry or shape. Such as the geometry of sp3 hybridization is tetrahedral which is of electron-precise molecule methane. The non-electron-precise molecule has a different geometry and shape. Such as the geometry of ammonia and water is tetrahedral but the shape is pyramidal and bent respectively.
