Question
Question: Which of the following hexoses will form the same osazone when treated with excess phenylhydrazine? ...
Which of the following hexoses will form the same osazone when treated with excess phenylhydrazine?
A ) D-glucose, D-fructose and D-galactose
B ) D-glucose, D-fructose and D-mannose
C ) D-glucose, D-mannose and D-galactose
D ) D-fructose, D-mannose and D-galactose
Solution
Identify the similarities/ differences between configurations for first two carbon atoms for the given hexoses since phenyl hydrazine only affects the configuration of first two carbon atoms.
Complete step by step answer:
Write the structures of given hexoses as follows:
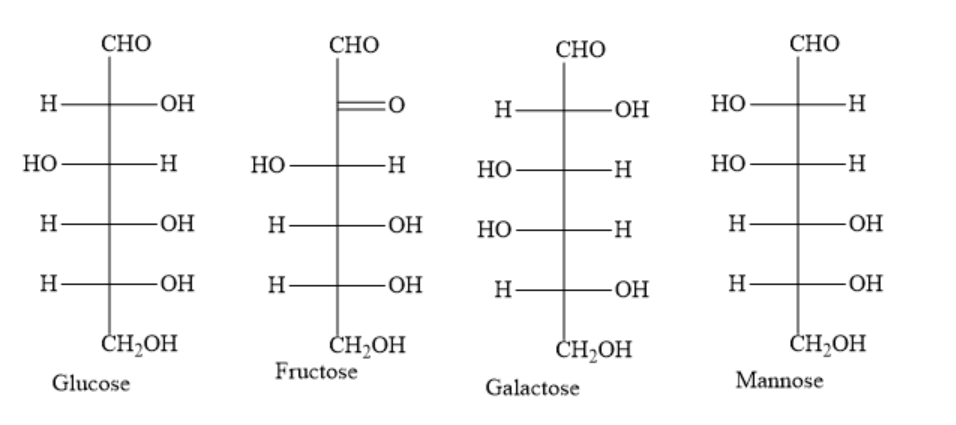
During the formation of osazone, only the first two carbon atoms participate. The remaining four carbon atoms remain as such.
D−glucose, D−fructose and D−mannose have different configurations only at second carbon atom. For the remaining carbon atoms, they have the same configuration.
In the Fischer projection formula for D-glucose, the hydroxyl group of the second carbon atom is on the right hand side. In the Fischer projection formula for D-mannose, the hydroxyl group of the second carbon atom is on the left hand side. D-fructose has a carbonyl group on the second carbon atom.
D−glucose, D−fructose and D−mannose form the same osazone when treated with excess phenylhydrazine.
The structures of the osazone from D−glucose, D−fructose and D−mannoseand D-galactose are as shown below:
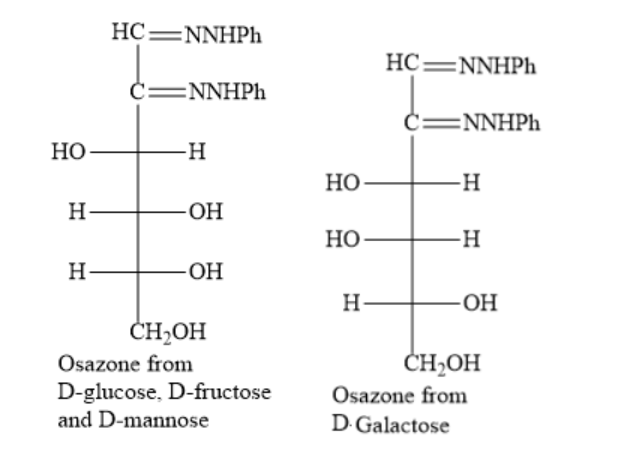
Hence, the option B) D−glucose, D−fructose and D−mannose is the correct option.
Note:
When two reactions are added, the values of the enthalpy changes are also added. When two reactions are subtracted, the values of the enthalpy changes are also subtracted. When a reaction is divided with a number, the enthalpy change value is also divided with the same number.
