Question
Question: Which of the following have zero dipole moment? A.p – Dichlorobenzene B.benzene – 1,4 – diol C...
Which of the following have zero dipole moment?
A.p – Dichlorobenzene
B.benzene – 1,4 – diol
C.Fumaric acid
D.Maleic acid
Solution
An arrangement of two equal and opposite charges separated by a finite distance is called an electric dipole. Electric dipole moment of an electric dipole is defined as the product of either of its charges and the length of the dipole.
Complete step by step solution:
Before we move forward with the solution of this question, let us understand some basic important concepts.
As discussed above, dipoles would be formed if there are equal and opposite charges placed at a finite distance, then we would form a dipole. But we must remember that the magnetic field caused by these charges is a vector quantity, this means that for having a net effective magnetic field, along with the magnitude of the charge of the particle, the direction in which it will be directed is also important. Mathematically, the relation for calculating the dipole moment can be given as follows:
Dipole moment = (charge) (distance between the charges)
If two equal and opposite charges are placed exactly across each with, with no other magnetic hindrance, then these two particles would cancel out their charges and would result in a net zero dipole moment.
To determine which compound has zero dipole moment, we must study their respective molecular structures.
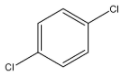
p – Dichlorobenzene
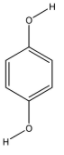
benzene – 1,4 – diol
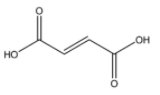
Fumaric acid
Maleic acid
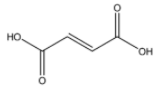
From the above structures, we can observe that only p – Dichlorobenzene has the capacity to cancel out the charges on its constituents, because both the chlorine atoms are placed exactly opposite each other.
Hence, Option A is the correct option.
Note:
Para-dichlorobenzene is an active ingredient of mothballs, deodorizers and fumigants. Due to the easy availability of this chemical, there is a considerable risk for accidental or intentional toxic exposure.
