Question
Question: Which of the following have planar structure? This question has multiple correct options A.\({I_...
Which of the following have planar structure?
This question has multiple correct options
A.I3−
B.H2O
C.PCl5
D.XeF4
Solution
Each molecule exhibits different geometry with respect to their bonding. Hybridization is the method used to determine the geometry. To check which molecule exhibits which geometry we will check for each.
Complete answer:
Let’s consider the first molecule I3−, this molecule exhibits linear geometry. There are three iodine atoms paired together, one atom has a negative charge due to which there are 3 lone pairs. Thus, the orientation will be linear with each atom at 180∘ angle with one another.
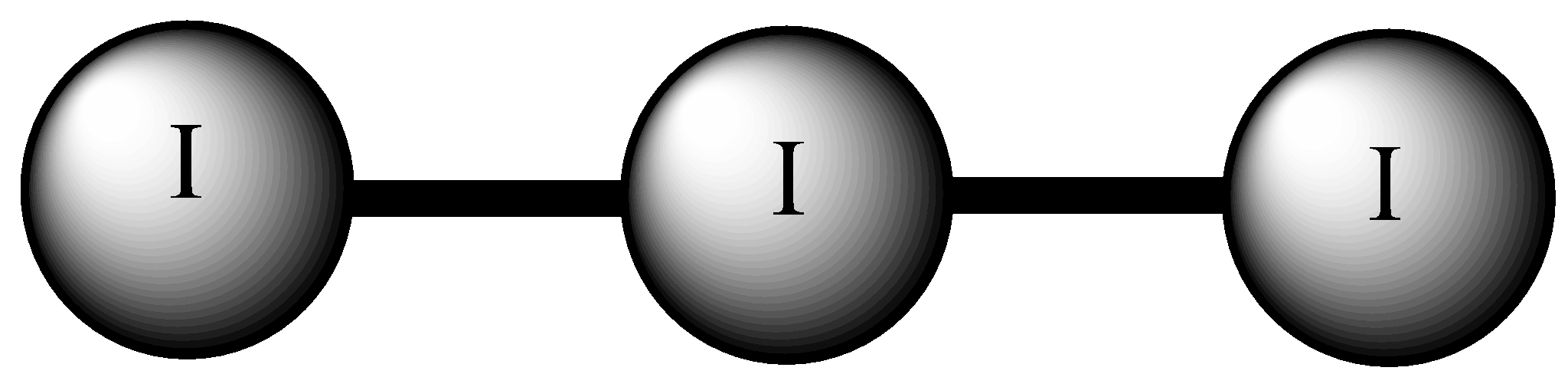
Thus, this is one correct answer.
Let us consider the next molecule is H2O. Water molecules have 4 electron density, with 2 bonded pairs and 2 lone pairs. Hence, it exhibits in bent shape and not planar. The geometry can be visualised as below,
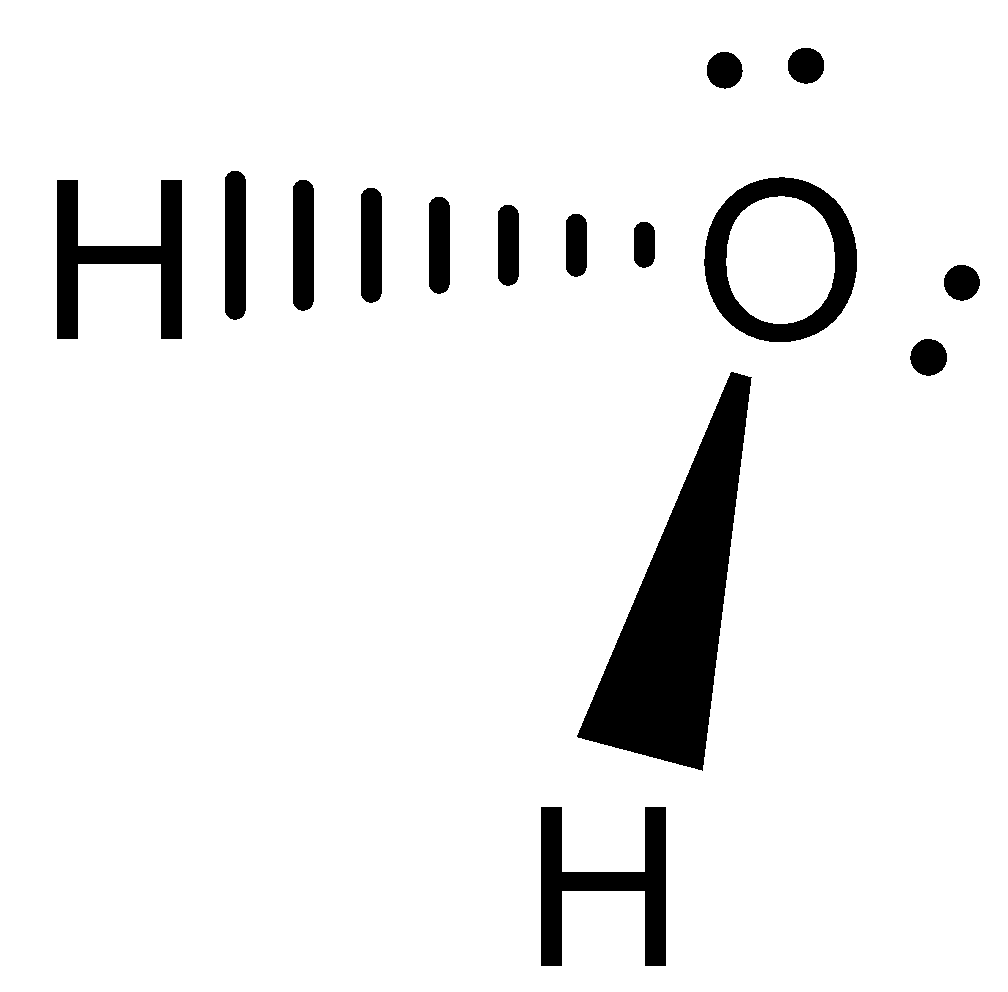
In the case of PCl5, phosphorus atoms are bonded with 5 chlorine atoms. It exhibits a trigonal bipyramidal structure which is not planar, with three atoms in the planar structure and one above and one below at the tip of the hedral structure.
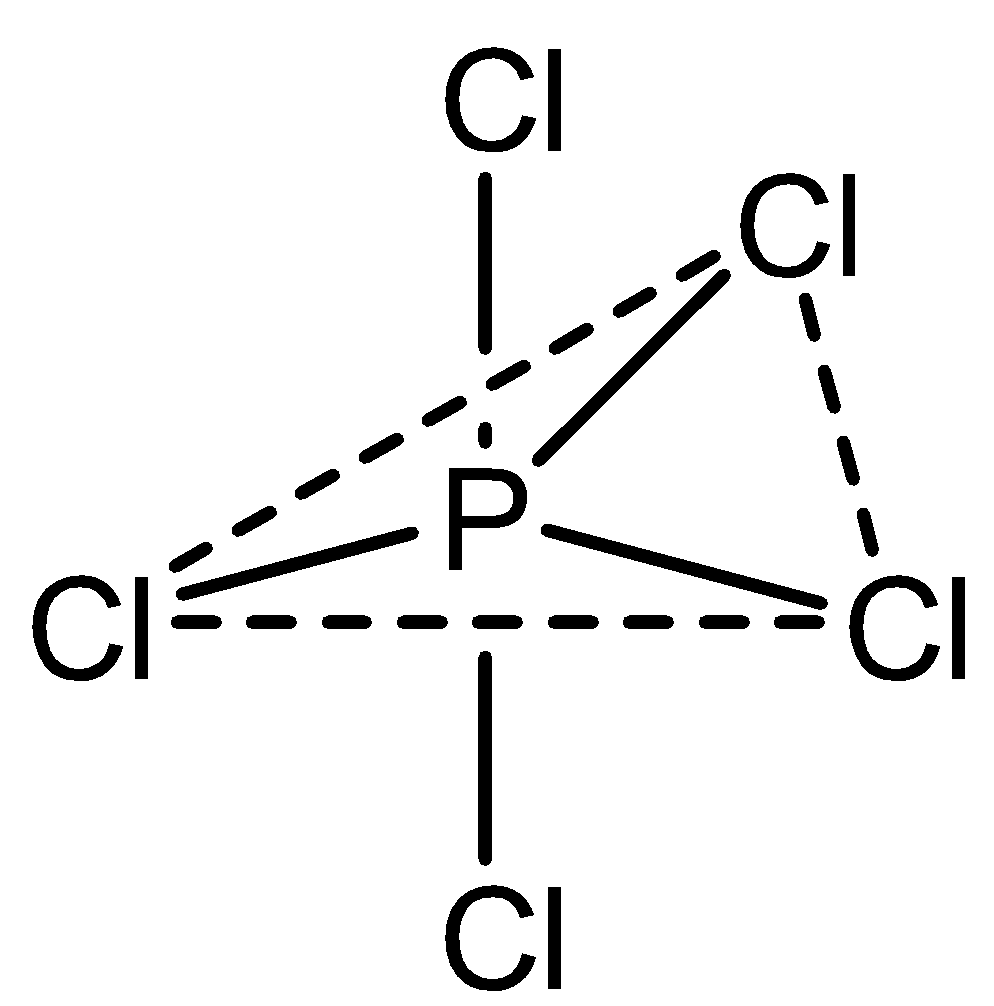
In the case of XeF4 which exhibits sp3d2 hybridization but in a square planar geometry with four fluorine atoms at the corner of the square plane and two lone pairs. Thus, the structure can be shown as,
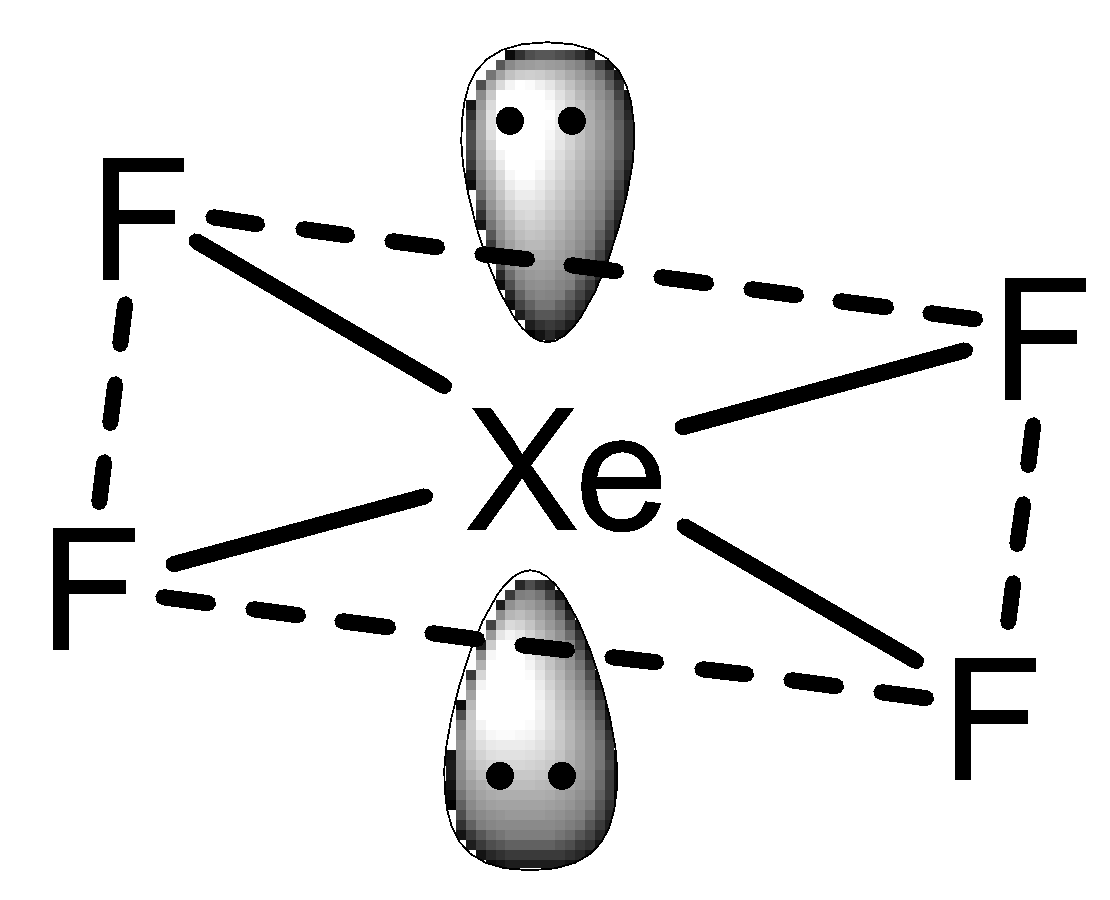
Thus, the correct answer to the question is option A and D.
Note: We have to remember that the hybridization depends on the bonding of an atom with respect to the molecules present. Some atoms carry lone pairs even after bonding like oxygen. Therefore, their structures get modified due to the presence of lone pairs. As they tend to repel from each other due to a strong negative charge. In questions like this, it is important to determine the structure of each molecule.
