Question
Question: which of the following have non-zero dipole moments? (A) \( S{F_4} \) (B) \( S{F_6} \) (C) \...
which of the following have non-zero dipole moments?
(A) SF4
(B) SF6
(C) XeO2F2
(D) SO3
Solution
Hint : Dipole moment is the measure of polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges. So, polarity is nothing but a separation of electric charge leading to a molecule having an electric dipole moment.
Complete Step By Step Answer:
The dipole moment is represented by μ (mue).
Dipole moment is given by
μ=δd
Where δ -charge ( δ+ and δ− )
d-distance between partial charges.
The unit of μ is debye(D).
SF4 molecule – name of the molecule is sulphur tetrafluoride. The hybridization type of the molecule is sp3d hybridization. The molecular geometry is trigonal bipyramidal. It contains four bond pairs and one lone pair.
It has a see-saw shape.
(all diagrams are drawn using paint 3d)
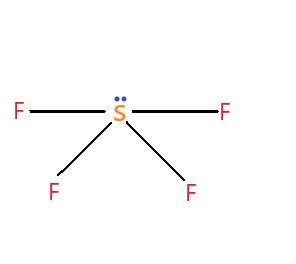
The molecular geometry of the SF4 molecule is not linear or any geometrical shape that will cancel out the partial negative charges on the fluorine, so the overall molecule is polar which makes it have a dipole moment of around 0.632D. So it has a non-zero dipole moment.
SF6 molecule – name of the molecule is sulphur hexafluoride. The molecule have sp3d2 hybridization. The molecular geometry is octahedral.
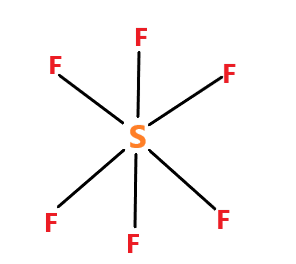
The SF6 molecule has no dipole moment because each S−F bond dipole is balanced by one of equal magnitude pointing in opposite direction of the other side of the molecule. So the net charge of the molecule is zero.
XeO2F2 molecule – the molecule name is xenon dioxide difluoride. The molecule have sp3d hybridization. The molecular geometry is trigonal bipyramidal.
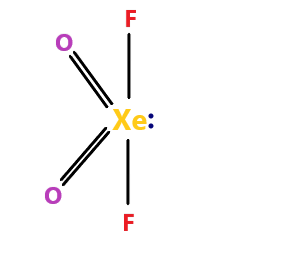
The molecule is polar. It has 4 bond pairs and 1 lone pair. Since this molecule has a lone pair, it is not symmetrical and therefore, has a net polar moment.
SO3 molecule – name of the molecule is sulphur trioxide. The molecule have sp2 hybridization. The molecular geometry is trigonal planar.
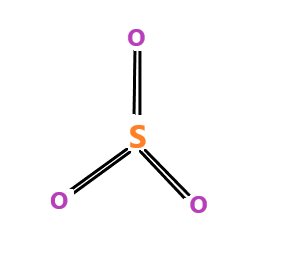
SO3 is a planar molecule with three bond angles 120 degrees apart. The individual dipole moments of S−O bond cancel out each other due to which the dipole moment of the molecule is zero.
Hence the option (A) and (C) have non zero dipole moments.
Note :
from dipole moment
-Ionic characters can be calculated.
-Geometry of the molecule can be predicted.
-Nature of the molecule can be predicted.
Keep in point that the dipole moment is a vector quantity.
