Question
Question: Which of the following have more resonance structures? A. Benzene B. Naphthalene C. Anthracene...
Which of the following have more resonance structures?
A. Benzene
B. Naphthalene
C. Anthracene
D. Phenanthrene
Solution
The resonance structures of the organic compounds is going to depend on the number of double present it and number of aromatic rings present in it. As the number of conjugated double bonds increases the number of resonance structures can increase.
Complete answer:
- In the question it is to find the organic compound which has a high number of resonance structures among the given options.
- Coming to given options. Option A, benzene.
- The resonance structures of the benzene are as follows.
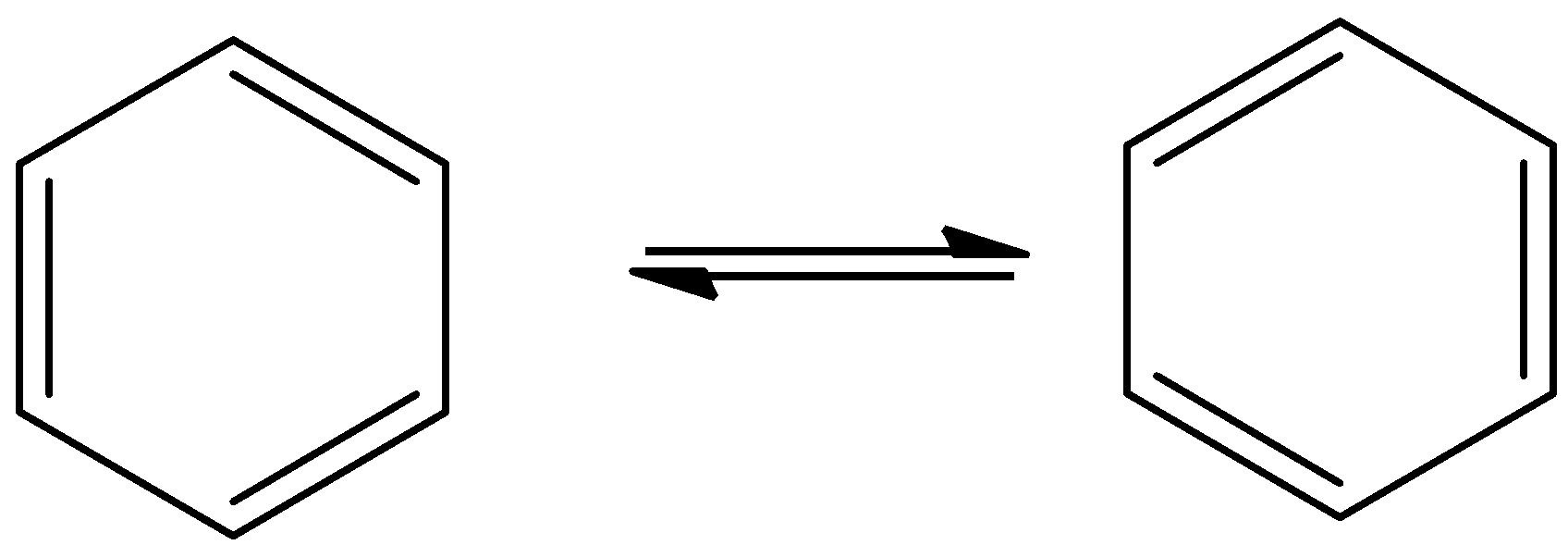
- Benzene can show two resonating structures.
- Coming to option B, Naphthalene.
- The resonance structures of the Naphthalene are as follows.
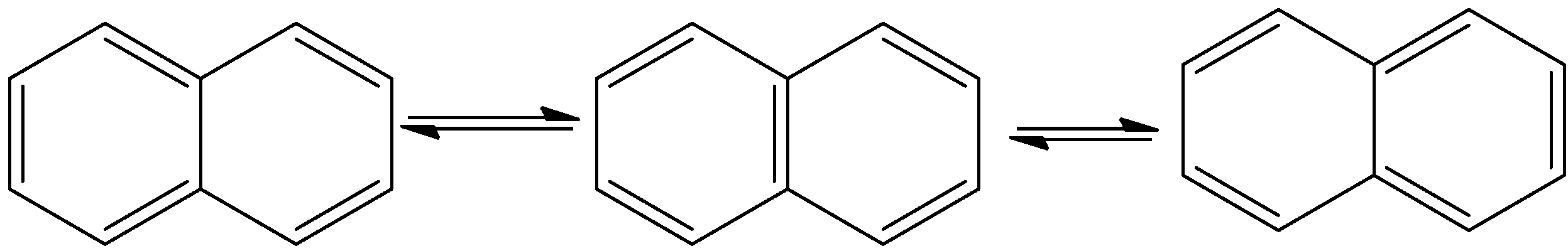
- Means naphthalene shows three resonating structures.
- Coming to option C, Anthracene.
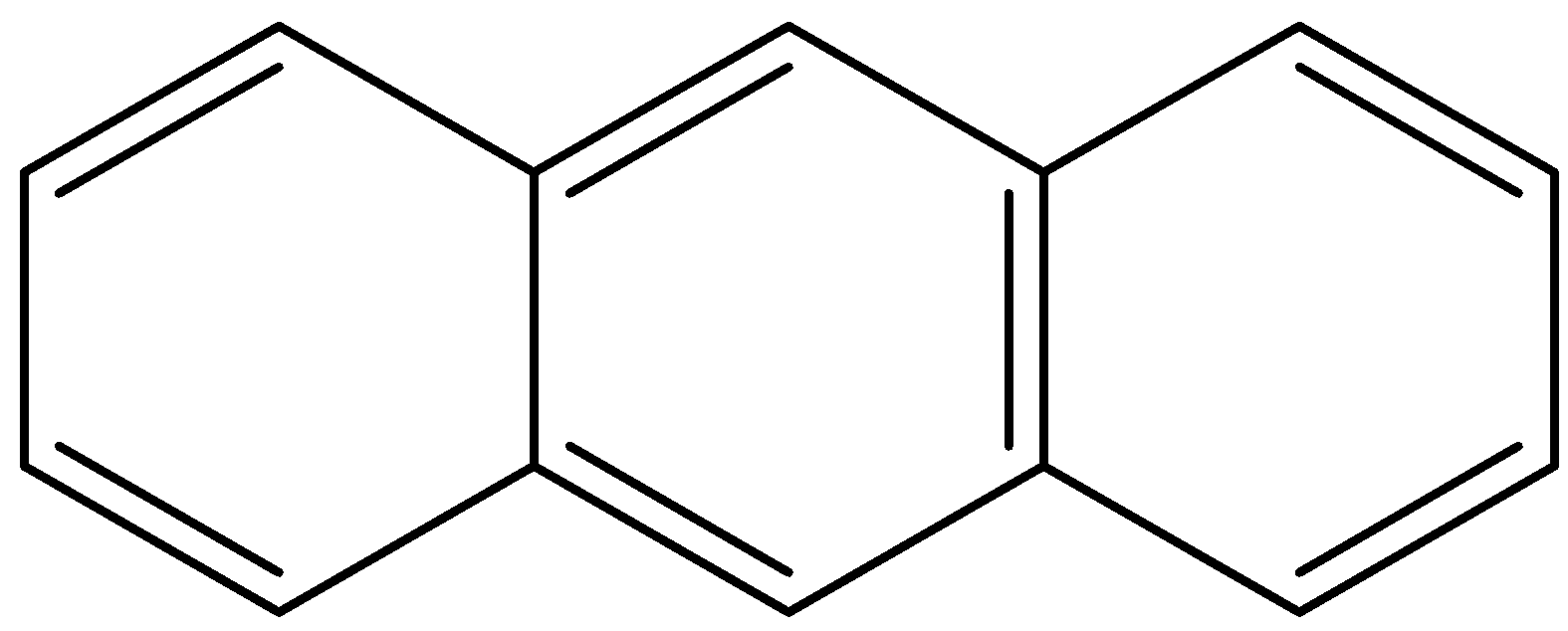
- The resonance structures of the Anthracene are as follows.
- Means anthracene shows three resonating structures.
- Coming to option D, Phenanthrene.
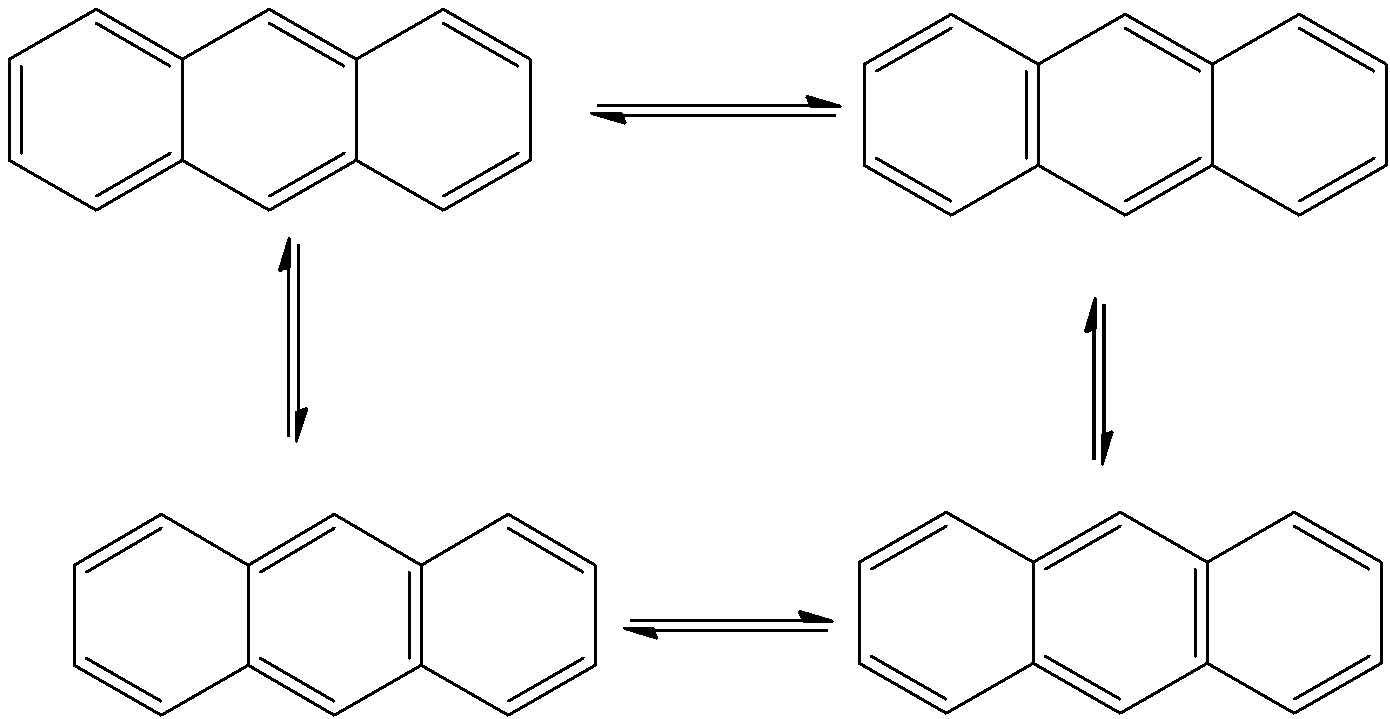
- The resonating structures of Phenanthrene are as follows.
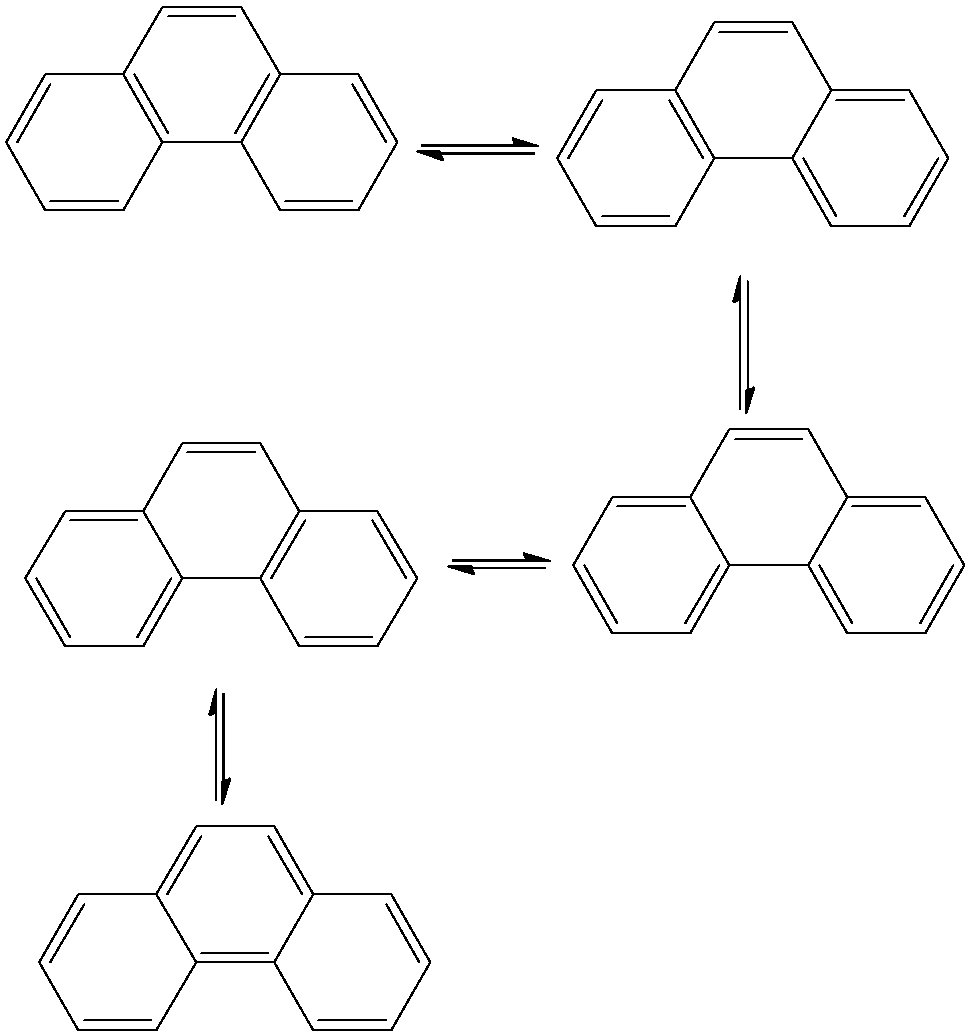
- Therefore Phenanthrene shows five resonating structures.
- Among the given options Phenanthrene shows the highest number of resonating structures.
So, the correct option is D.
Note:
The electrons which are present in the pi bond are going to resonate in the aromatic rings to get more stability of the organic compounds. The reason behind the stability of the organic compounds is the number of resonating structures. The number of resonating structures is going to decide the stability of the particular aromatic molecule.
