Question
Question: Which of the following have +M effect (\({e^ - }\) donating mesomeric effect) ? This question has ...
Which of the following have +M effect (e− donating mesomeric effect) ?
This question has multiple correct options
(A) NO2
(B) COOH
(C) NH2
(D) SR
Solution
The mesomeric effect in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecule by the interaction of two Pi(π) bonds or between a pi bond and lone pair of electrons present on an adjacent atom.
Complete answer:
There are two types of mesomeric effect:
a) +M effect (e− donating effect)
b) −M effect (e− withdrawing effect)
1. +M effect (e− donating effect) – When the electrons or the π electrons are transferred from a particular group towards a conjugate system, this increasing the electron density of the conjugate system then such a phenomenon is known as (+M) effect e− donating mesomeric effect. For the +M effect the group should have a lone pair or negative charge.
Group showing +M effect –
−NH,−NH2−NHR,−NR,−O,−OH,−OR,−F,−Cl,−SH,−SR etc.
2. −M effect (e− donating effect) – When pi-bond electrons are transferred from the conjugate system to a particular group thus the electron density of the conjugate system is discovered, then the phenomenon is known as negative isomeric effect (−M).
Group showing −M effect –
−NO2,−CN,−COX,−SO3H,−CHO,−CONH2,−COR,−COOH,−COOR etc.
Therefore from the above information
−NO2 and −COOH are electron withdrawing groups
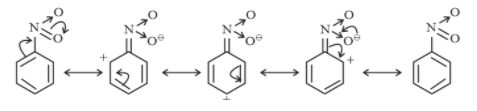
Resonating structures of nitrobenzene
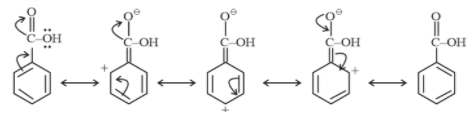
Resonating structure of Benzoic acid
From above structures, it is clear that −NO2 and −COOH withdraw electrons from the ring. So they show a −M effect.
−NH2 and −SR are electron donating groups –
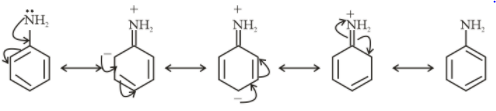
Resonating structures of Aniline
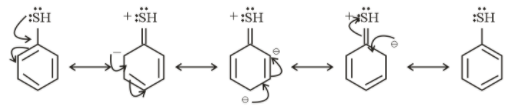
Resonating structure of Thiophenol
From the above structure, it is clear that −NH2 and −SH donate electrons in the ring. So, they show +M effect.
Hence, the correct answers are (C) and (D) (−NH2,−SR).
Note: Molecules which have double or triple bond show −M effect whereas molecules which have lone pair on the directly bonded atom show +M effect
