Question
Question: Which of the following have lowest degree of covalency?...
Which of the following have lowest degree of covalency?
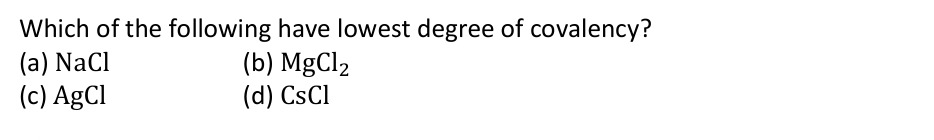
NaCl
MgCl2
AgCl
CsCl
CsCl
Solution
To determine which compound has the lowest degree of covalency, we need to consider Fajan's rules, which predict the degree of covalent character in an ionic bond. Lower covalent character corresponds to higher ionic character.
Fajan's rules state that covalent character increases with:
- Smaller cation size.
- Larger anion size (the anion is Cl- in all options, so this factor is constant).
- Higher charge on the cation.
- Cations with pseudo-noble gas configuration (like Ag+, Cu+) compared to cations with noble gas configuration (like Na+, K+, Mg2+).
Based on Fajan's rules, the order of increasing covalent character is predicted to be:
CsCl (largest cation, +1 charge, noble gas config) < NaCl (smaller cation than Cs+, +1 charge, noble gas config) < MgCl2 (smaller cation than Na+, +2 charge, noble gas config) < AgCl (+1 charge, pseudo-noble gas config)
Therefore, CsCl is predicted to have the lowest degree of covalency (highest ionic character).
