Question
Question: Which of the following has zero dipole moment ? A. \(B{F_3}\) B. \(BeClBr\) C. \(C{H_2}C{l_2...
Which of the following has zero dipole moment ?
A. BF3
B. BeClBr
C. CH2Cl2
D. COS
Solution
Dipole moment is defined as the product of the magnitude of the charge and the distance between the centre of positive and negative charges. Dipole moment is a measure of the polarity of a molecule .
Complete step by step answer:
Dipole moments arise from electronegativity differences .
it is denoted by μ
μ=q×r
where , q=magnitude of charge
and r= distance between charges
In the case of polyatomic molecules, μ not only depends upon the individual dipole moments of the bonds but also on the spatial arrangement of various bonds in the molecule.
When all the bonds in a molecule are oriented in such a way that they totally cancel each other's effects then the dipole moment is said to be zero.
In case of BF3,
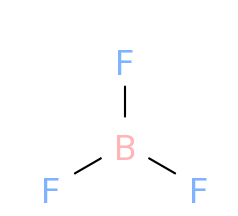
As we can see , the molecule has a trigonal planar shape
That is, the bonds are oriented at an angle of 120∘ such that the resultant dipole due to two B−F bonds cancel the third bond dipole and so the net dipole moment becomes zero.
In all the other molecules given in the options , the bonds are so arranged that the net dipole moment is not equal to zero
So, the correct answer is Option A .
Note:
Dipole moment is used to calculate the polarity of the bonds . The dipole moment of a molecule depends on two things: the individual dipole moment of each bond and the arrangement of the molecules .
