Question
Question: Which of the following has the shortest carbon-carbon bond length? A. Benzene B. Ethane C. ...
Which of the following has the shortest carbon-carbon bond length?
A. Benzene
B. Ethane
C. Ethyne
D. All have same length
Solution
We know that the number of bonds between a pair of atoms in a molecule indicates its bond order. In oxygen (O=O) molecule, there are two bonds, so the bond order of oxygen molecule is two, in nitrogen (N≡N) molecule, bond order is three.
Complete step by step answer:
Let’s discuss bond length in detail. It is the distance between centres of two atoms which are bonded covalently. Bond length is dependent on the bond order. Greater the bond order, the pull between two atoms is stronger which results in shorter bond length. Therefore, increase of bond length in the increasing order is triple bondLet’s identify the correct answer from the options.
Option A is Benzene, option B is ethane and option C is ethyne.
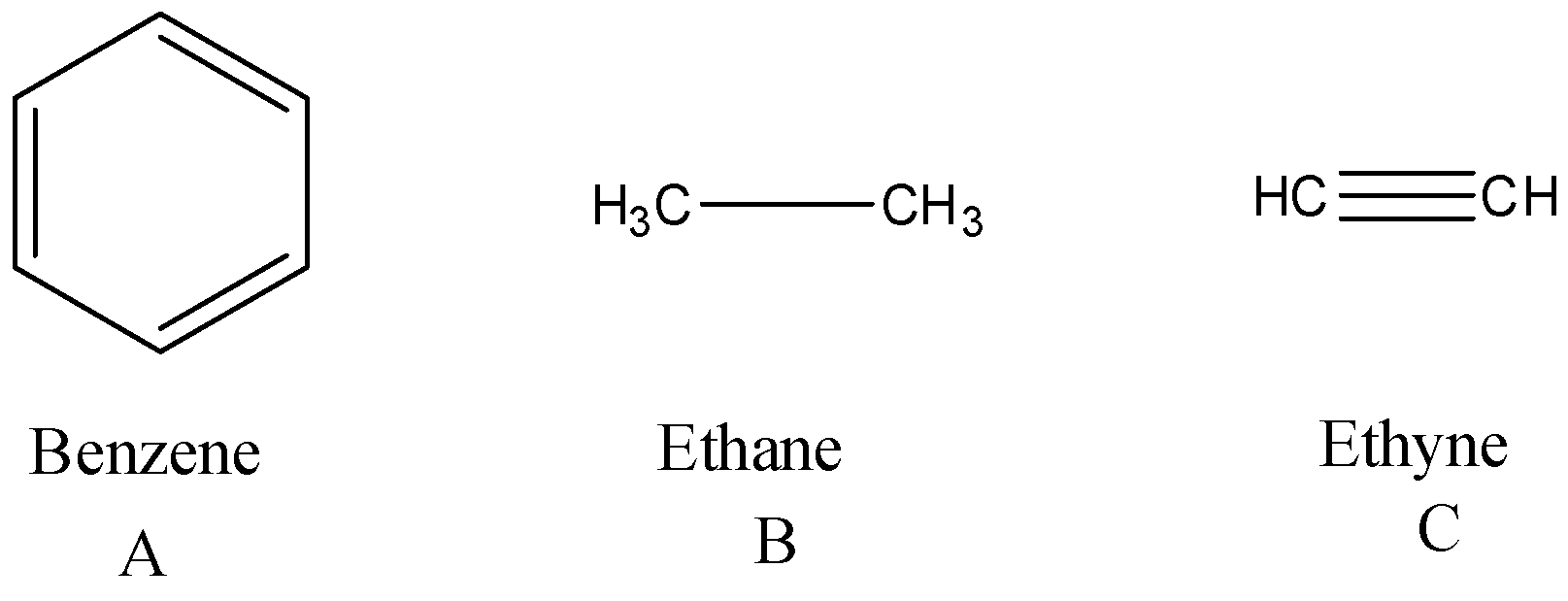
We know that bond order is inversely proportional to bond length. That means, more the bond order, shorter the bond length. Here, ethyne has a triple bond, benzene has a double bond and ethane has a single bond. So, the compound whose bond length is smallest is ethyne as it has bond order 3. Benzene has more bond length than ethyne as it has bond order 2 and the highest bond length found is ethane as it has bond order 1. So, the order of increasing bond length is ethyneHence, the correct answer is option C.
Note:
There is formula to calculate bond order of molecule, that is,
Bondorder=2NA−NB
Here, NA represents number of electrons in antibonding molecular orbital and NB
represents the number of electrons in a bonding molecular orbital.
