Question
Question: Which of the following has the most acidic hydrogen? A.
B.
C.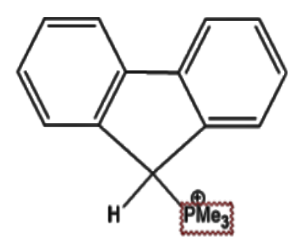
D.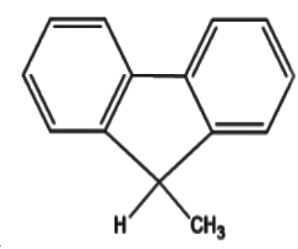
Solution
The most common understanding is that strong acids have relatively stable conjugate bases.The more stable the base stronger will be the acid thus formed.Although the examination of pKa values offers insights into the acidic trends.By using theoretical guidelines too we can estimate the acidity of compounds.
Complete answer: 1)The acidity of hydrogen atoms can be determined with the formation of a stable base.
2)For cyclic compounds the acidity of hydrogen atoms is determined with the stability of resonance hybrids which will eventually form.
3)The presence of strong electron withdrawing groups in the cyclic ring will further stabilise the compound.
4)In all of the options,option (a) lacks any electron withdrawing group attached to the carbon atom which has the hydrogen atom.Hence this option is incorrect.
5)In option (d) the methyl group present is not a strong electron withdrawing group as compared to the groups present in options (b) and option (c).Thus we can safely conclude that option (b) is incorrect.
6)Thus we are left with two options.For this we need to analyse the electron withdrawing capacity of these two groups.
7)The electron withdrawing group N⊕Me3 is a stronger electron withdrawing group as compared to P⊕Me3 hence it will produce a more acidic hydrogen as compared to the latter.This is also due to the fact that N⊕Me3 will aid in the stability of the resonance hybrid which will eventually form.
With these points in consideration we conclude that option (B) is correct.
Note:
There are various factors which we need to consider for the formation of acidic hydrogen. Factors like stability of conjugate base,presence of electron withdrawing groups affect the formation of acidic hydrogen greatly.
