Question
Question: Which of the following has the lowest \(p{{K}_{a}}\) value? (A)  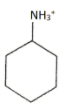
(B) 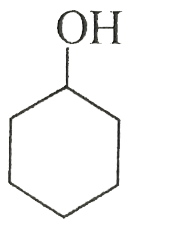
(C) 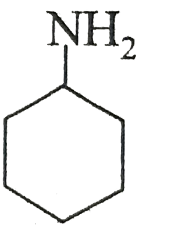
(D) 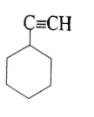
Solution
The pKa value is used to indicate the strength of an acid. pKa is the negative of the log of acid dissociation constant or we can say that the Kavalue. A lower pKa value indicates a stronger acid.
Complete step by step solution:
pH represents the basicity or the acidity of a solution on a logarithmic scale on which in value of 7 is considered to be a neutral value i.e. it is neither acidic nor basic. If the value is greater than 7 then it is basic and if the value is less than 7 than it is acidic. After removal of the hydrogen ion the compound which makes the highly stable carbocation. The more stable carbocation is more acidic and the compound which is more acidic will have low pKa value. In the first compound there is -NH3+ in the second compound there is -OH group in the third compound there is - NH2 group and in the fourth compound the group present is -C≡CH. Group which is more electron withdrawing will easily donate the H+ion. The most electron withdrawing group is -NH3+ because it easily donate the H+ ion and become a neutral compound hence it will be most acidic among all the given options and have the least value of pKa.
Hence the correct answer is option (A)
Note: Strong acid and base will have low pKa value whereas the weak acid and base will have high pKa value. Acids are having pH less than 7 and the bases will have pH more than 7.
