Question
Question: Which of the following has the lowest heat of combustion? A)  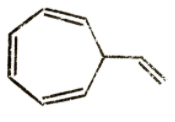
B) 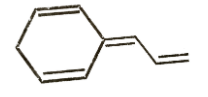
C) 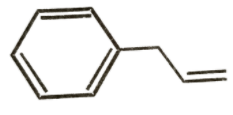
D) 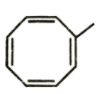
Solution
Carbon number is the same in all options. So, look at the stability. More stable the compound, the less is its heat of combustion. The stability is related to factors such as resonance, bond strength, and bond length.
Complete answer:
The heat of combustion is the heat energy released when a hydrocarbon combines with oxygen to form carbon dioxide and water along with lots of heat energy. The formation of new bonds releases energy while the breakage of bonds requires energy. So, the compound in which energy required to break the bonds is the most, will have the lowest heat of combustion because it is the difference of the energy released by bond formation and the energy required for bond breakage.
The carbon numbers are the same in all the options. So, we have to look for the most stable structure. The ring structures that have alternate double bonds may have an aromatic structure (provided they obey Huckel rule of 4n + 2π -electrons ) which is more stable than aliphatic, cyclic, or allylic structures.
It is clear that options A and B do not have aromatic structures and are relatively unstable. Option C has 6π electrons which is a Huckel number as it can be expressed in the form of 4n + 2. So, option C is the most stable compound and will require greater energy for bond breakage than the other two compounds. So, its net energy release as heat of combustion will be lowest.
So, the structure shown in option C will have the lowest heat of combustion.
Note: Normally, the higher carbon-number compound does have the high heat of combustion and vice-versa, but here the carbons number is the same. So, look for the stable and unstable structures.
