Question
Question: Which of the following has the highest boiling point? (A)- \({{C}_{3}}{{H}_{7}}Cl\) (B)- \({{C}_...
Which of the following has the highest boiling point?
(A)- C3H7Cl
(B)- C4H9Cl
(C)- CH3CH(CH3)CH2Cl
(D)- (CH3)3C−Cl
Solution
Hint: The boiling point of halogen containing organic compounds depends on the molecular weight of the compound and the structure of the compound. As branching in a molecule increases, the molecule becomes more compact and the boiling point decreases.
Complete step by step solution:
Let’s look at the answer of the given question:
If we compare the number of carbon atoms present in all the options then 4 is the maximum number. So, in option (A) only 3 carbon atoms are present. So, it will have lower boiling point than the other given compounds.
In halogenated hydrocarbons as the number of branches increases, then the boiling point of the molecule decreases. This is because the shape of the molecule becomes more compact. Due to this the vapour pressure of the molecule increases and the boiling point of the molecule is decreased.
In option (C), the given compound has 1 branched substituent. So, its molecule will become compact. Hence, it will have lower boiling point.
In option (D), the given compound is t-butyl chloride. It has two branched substitutes. The molecule is almost spherical in shape. Due to this the vapour pressure of the molecule will be very high and its boiling point will be very low.
In option (B), the given compound is n-butyl chloride. It has a maximum number of carbons and it has no branched substituent. The molecule will have low vapour pressure and high boiling point.
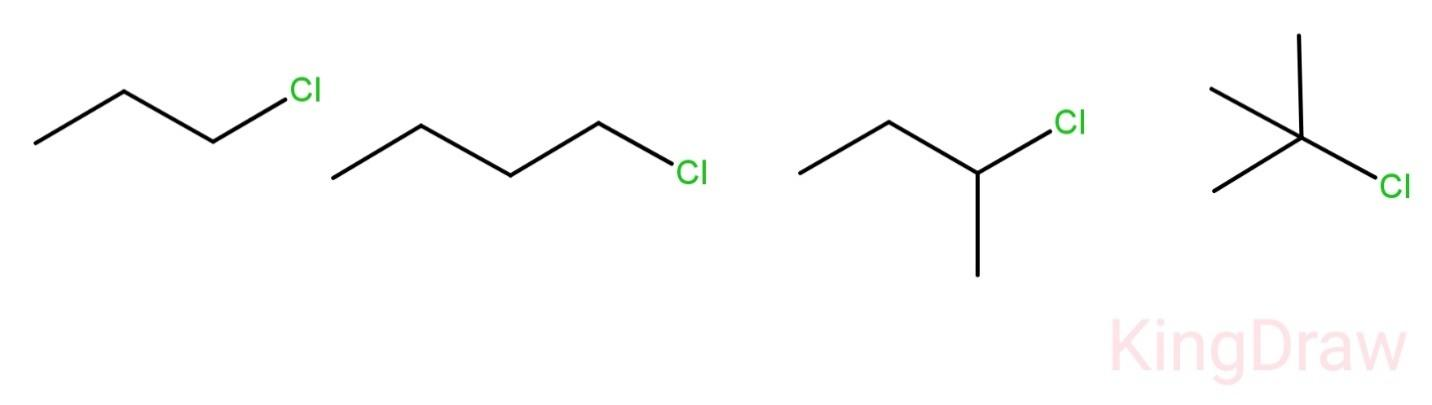
So, we have seen that among the given compounds n-butyl chloride has the highest boiling point.
Hence, the answer of the given question is option (B)
Note: The boiling point of halogenated hydrocarbons is greater than the corresponding hydrocarbons. This is because of dipole-dipole interactions in halogenated hydrocarbons.
Halogens are electronegative elements and they have a tendency to withdraw electron density. So, they develop a partial negative charge on them and the other atom develops the partial positive charge. Because of this a dipole is developed in the molecule and dipole-dipole interactions are generated.
