Question
Question: Which of the following has the greatest number of atoms? A. \({\text{1}}\,{\text{g}}\) of butan...
Which of the following has the greatest number of atoms?
A. 1g of butane (C4H10)
B. 1g of nitrogen (N2)
C. 1g of silver (Ag)
D. 1g of water (H2O)
Solution
According to the Avogadro, one mole of any substance have 6.02×1023 atoms, ions, or molecules. This number 6.02×1023 is known as Avogadro number. The total number of atoms can be determined by multiplying the moles of a substance with Avogadro number.
Step by step answer: The number of moles in a substance is determined by using the mole formula which is as follows:
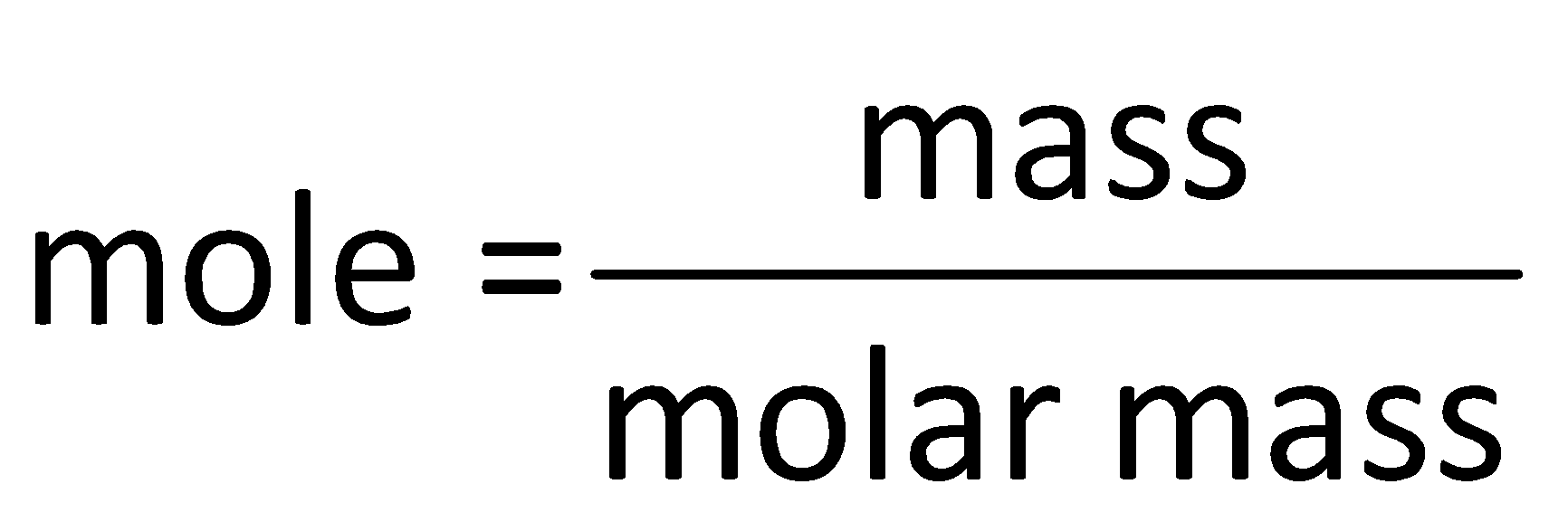
Determine the mole in 1g of butane as follows:
Substitute 1g for mass and 58g/mol for molar mass.
moleof C4H10 = 58 g/mol1 g
moleof C4H10 = 0.017mol
Multiply the mole of butane with Avogadro number to determine the total number of molecules.
Moleculesof C4H10 = 0.017mol×6.02×1023molecules/mol
Moleculesof C4H10 = 1.04×1022
So, the number of molecules in 1gof butane is 1.04×1022.
One molecule of butane has 14atoms. So, the total number atoms are as follows:
1.04×1022×14
14.6×1022atoms
So, the number of atoms in 1g of butane is14.6×1022.
Determine the mole in 1g of nitrogen as follows:
Substitute 1g for mass and 14g/mol for molar mass.
moleof N2 = 14 g/mol1 g
moleof N2 = 0.071mol
Multiply the mole of nitrogen with Avogadro number to determine the total number of molecules.
moleculesof N2 = 0.071mol×6.02×1023molecules/mol
moleculesof N2 = 4.3×1022
So, the number of molecules in 1g of nitrogen is 4.3×1022.
One molecule of nitrogen has 2atoms.
=4.3×1022×2
=8.6×1022
So, the number of atoms in 1g of nitrogen is 8.6×1022.
Determine the mole in 1g of silver as follows:
Substitute 1g for mass and 108g/mol for molar mass.
moleof Ag = 108g/mol1 g
moleof Ag = 9.259×10−3mol
Multiply the mole of silver with Avogadro number to determine the total number of molecules.
moleculesof Ag = 9.259×10−3mol×6.02×1023molecules/mol
moleculesof Ag = 0.55×1022
One molecule of silver has one atom.
Atomsof Ag = 1×0.55×1022
Atomsof Ag = 0.55×1022
So, the number of atoms in 1g of silver is 0.55×1022.
Determine the mole in 1g of water as follows:
Substitute 1g for mass and 18g/mol for molar mass.
moleof H2O = 18g/mol1 g
moleof H2O = 0.055mol
Multiply the mole of water with Avogadro number to determine the total number of molecules.
moleculesof H2O = 0.055mol×6.02×1023molecules/mol
moleculesof H2O = 3.3×1022
So, the number of molecules in 1g of water is 3.3×1022.
One molecule of water has 3atoms.
No. of atoms =3×3.3×1022
No. of atoms =9.9×1022atoms
So, the number of atoms in 1g of water is9.9×1022.
1g of butane has the highest number of moles so, option (B), (C), (D) are incorrect.
Therefore, option (A) 1g of butane(C4H10), is correct.
Note: Atomicity also denotes the number of atoms in a substance. Here, the gram amount of each substance is given so, the total number of atoms is determined by multiplying the moles with Avogadro's number and number of atoms in one molecule. If the gram amount is not given the total number of atoms can be determined by adding the number of atoms of a substance.
