Question
Question: Which of the following has the chemical formula \({{C}_{4}}{{H}_{10}}\)? A. Ethane B. Butane C...
Which of the following has the chemical formula C4H10?
A. Ethane
B. Butane
C. Isopropane
D. Pentane
Solution
To answer this, you should have an idea about IUPAC type of nomenclature. The name of any organic compound can be known by the number of carbon atoms it has, referred to as the prefix of that compound while the suffix says about the type of functional group or number of bonds present in the compound.
Complete step by step solution:
To answer this, you should have an idea about IUPAC type of nomenclature.
In order to name an organic compound, we should memorize a few basic names. In general, the base part or prefix of the name reflects the number of carbons in the parent chain while the suffix reflects the type of bond (single or double or triple bond) or type of functional group present in the parent chain.
So, for the following number of carbon atom in a straight saturated hydrocarbon compound, the name should be:
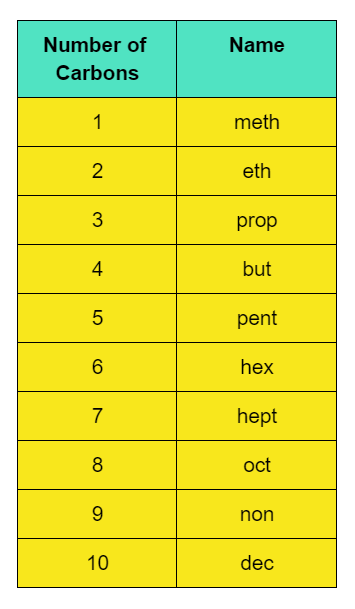
While, for the number of carbon-carbon bonds, the naming should be like:
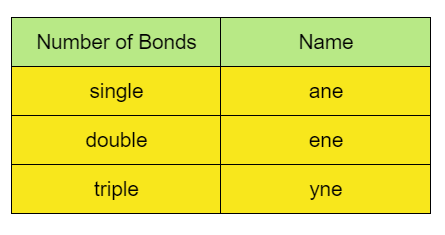
So, as per the formula C4H10,
H3C−CH2−CH2−CH3
The number of carbon atoms present are 4.
And the carbon atoms present are bonded with single bonds.
So, the prefix would be “but-”, as there are four carbon atoms whereas the suffix would be “-ane”, as the carbon atoms are bonded with single bonds.
Thus, the organic compound with formula C4H10, will be termed as butane.
Hence, the correct option is B.
Note: When naming organic compounds, you should remember the rules of IUPAC (International Union of Pure and Applied Chemistry) nomenclature. So, in order to give compounds a name, certain rules by IUPAC are followed. This is used to give a consistency to the names around the world.
