Question
Question: Which of the following has maximum energy? A.
B.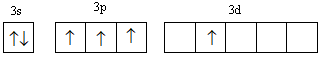
C.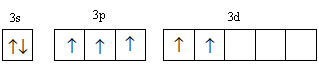
D.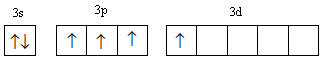
Solution
To answer this question we should know the rules of filling electrons in orbitals that are Pauli Exclusion Principle, Aufbau rule, and Hund’s rule. Remembering all these rules we will decide the orbital having the highest energy then we will check if the highest energy orbital has any electrons and if have then how many.
Complete step-by-step answer: We use three rules in filling of electrons.
Pauli rule: according to which two electrons cannot have the same value for all four quantum numbers.
Hund’s rule: according to this rule, electrons are filled singly first then get paired.
Aufbau rule: according to this electrons get filed in lower energy level first then in higher energy level.
The electrons in the orbital of lower energy are more stable, so we fill the electrons in lower energy orbitals first. Electrons in higher energy orbital cause the high energy of electronic configuration. More number of electrons in a high energy orbital further increases the energy.
The energy of 3s and 3p is lower than 3d-orbital, so electrons will get filled unpaired first and pairing will take place.
In configuration-A, two electrons get filled in s-orbital. Three electrons are filled unpaired first in p-orbital then one electron gets paired so this is a stable configuration because a higher energy orbital has no electrons.
In configuration-B, two electrons get filled in s-orbital. Three electrons are filled unpaired first then the fourth electron goes in the higher energy d-orbital so this configuration is somewhat less stable than configuration-A, so has higher energy than configuration-A.
In configuration-C, two electrons get filled in s-orbital. Three electrons are filled unpaired first then two electrons go in the higher energy d-orbital so this configuration is less stable than configuration-A, B and D hence has the highest among all four configurations.
In configuration-D, two electrons get filled in s-orbital. Three electrons are filled unpaired first then the fourth electron goes in the higher energy d-orbital so this configuration is somewhat less stable than configuration-A, so has higher energy than configuration-A.
So, configuration C has maximum energy.
Therefore, option (C) is correct.
Note: The energy of the orbital is determined by using n + l rule. The orbital having low value of n + l will have low energy hence more stable and hence it will get the electrons first. If two orbitals have the same value of n + l, the energy is lowest for the one having low value of l. according to n + l rule, here, the energy order is 3s < 3p < 3d. So, 3s-orbital gets two electrons first. Then 3p-orbital gets three electrons after that again three electrons get filled in 3p-orbital that causes paring.
