Question
Question: Which of the following has intramolecular H-bonding? A. Ortho-nitrophenol B. Ortho-boric acid ...
Which of the following has intramolecular H-bonding?
A. Ortho-nitrophenol
B. Ortho-boric acid
C. Both (A) and (B)
D. None of these
Solution
Before solving this question, we should first know about Hydrogen-bonding, its types and then we can look at the options and find out which of them has intramolecular H-bonding. The formation of the hydrogen bond is Hydrogen bonding. They are of two types: Intermolecular and Intramolecular.
Complete answer:
Hydrogen- Bonding occurs because of the dipole-dipole interaction between the Hydrogen atom and a strongly electronegative element and another electronegative atom lies in the region of the Hydrogen atom having a lone pair of electrons. Let us now discuss Intramolecular Hydrogen Bonding.
Intramolecular H- Bonding: It is the bonding that occurs between the same molecule. It occurs in compounds which have two groups in which one group has a hydrogen atom joined to an electronegative atom and the other group has a strongly electronegative atom joined to a weak electronegative atom of another group.
Let us see the first option, In ortho-nitrophenol structure, the donor and the acceptor both are in the same molecule, so Intramolecular Hydrogen bonding is possible in this.
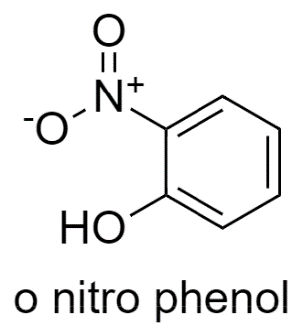
Now moving on to the second option, In ortho-boric acid structure, the donor and the acceptor are not within the same molecule, therefore it shows Intermolecular Hydrogen bonding.
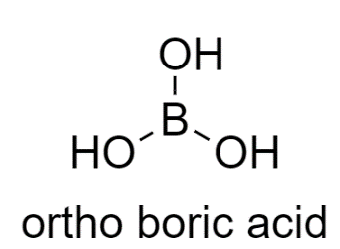
So, the correct answer is “Option A”.
Note:
There is another type of hydrogen bonding i.e. Intermolecular H- Bonding. It is the bonding that occurs between different molecules of the same or different compounds. Some examples of it are Hydrogen bonding in water, alcohol, etc.
