Question
Question: Which of the following has highest enol content. A.
B.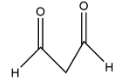
C.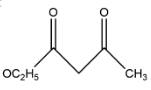
Solution
Enol content is the number of structures formed after taking one H from its alpha position to carbonyl oxygen. The compound which makes the highest number of enol structure possible, will have the highest enol content. You just have to see the number of hydrogen present at the alpha position. They will move from there and get connected to the oxygen, forming a double bond on the initial position.
Complete step-by-step answer: For the enol content, you have to see the number of alpha H so let’s start counting alpha H for respective structures. In the first we have two carbonyl group they will term as 0and the next carbon will be α so in this first option we have three alpha positions where two are having three hydrogens and one is having two hydrogens, a total of eight hydrogens are present so it will form eight enol structures.
In the second option we have only one alpha carbon atom, which is having only two hydrogens so let’s say that it will form only two structures and thus having two enol. In the third option we have two alpha positions in which one is having three hydrogens and one is having two hydrogens. The third carbon is having a group which is connected to oxygen.
Option A is correct.
Note: While forming the enol structure, in that process the hydrogen which will present on the alpha carbon tends to move from there and get connected to oxygen. The main thing is that you have to only count the number of alpha H and not the beta hydrogens because they don’t move to form enol. Enol is just form from alpha H.
