Question
Question: Which of the following has high resonance energy among the following pairs? A. \[C{H_3}COOH\] and ...
Which of the following has high resonance energy among the following pairs?
A. CH3COOH and CH3COONa
B. CH2=CH− and CH2=CH−OH
C.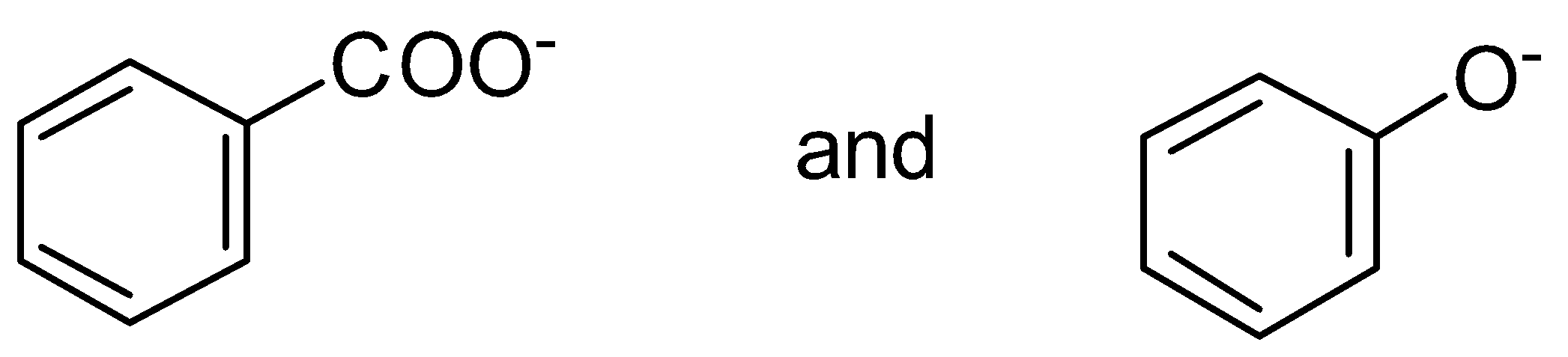
D. 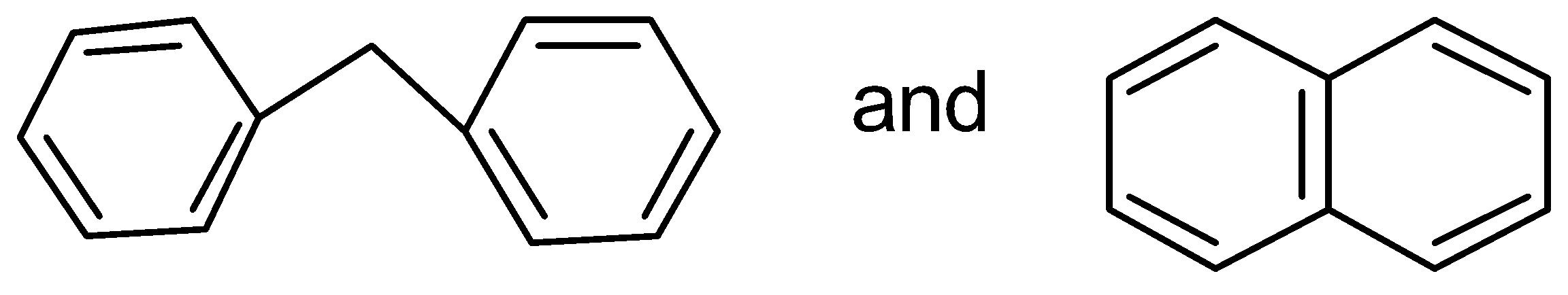
E. 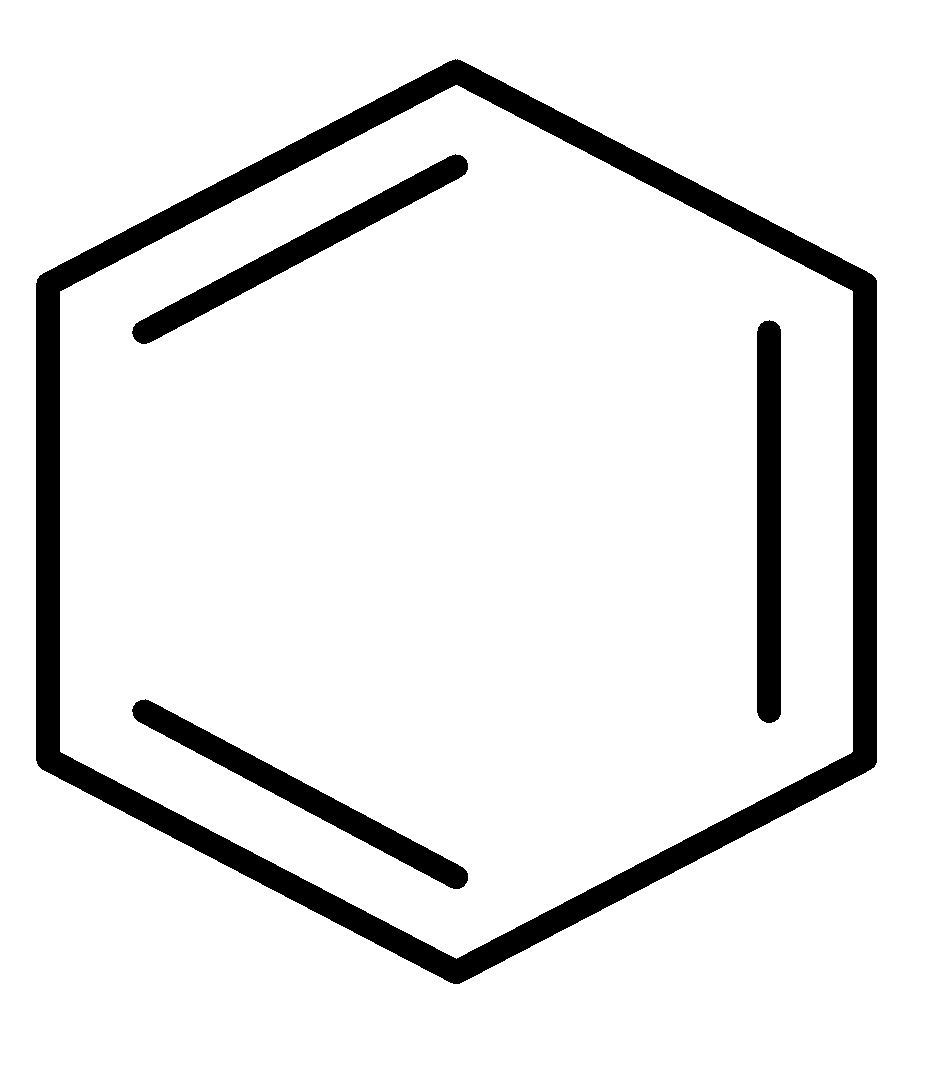 and CH2=CH−CH=CH−CH=CH2
and CH2=CH−CH=CH−CH=CH2
Solution
In order to answer this question, we should know about the concept of resonance energy. The resonance energy of a compound depends on its stability and aromaticity. For this question, we have to compare the aromaticity of the compounds given in pairs.
Complete answer:
Let’s completely understand the solution to this question.
First, let us know about the phenomenon named resonance. Resonance is the withdrawal or releasing effect of electrons attributed to a specific substituents through the delocalization of pi- electrons in organic compounds. This effect is resonance or mesomeric effect.
Here, in this question, we need to compare the resonance energy. The resonance energy is dependent on the stability and aromaticity of the compounds. Resonance energy is directly proportional stability of the compound as well as aromaticity.
Resonance energy is the difference in the energy between the most stable contributing structure for a compound and its resonance hybrid is resonance energy.
Let’s have a look at each pair one by one.
A. CH3COOH and CH3COONa
Here, CH3COONa has Na+cation which leaves this group and becomes CH3COO− . This negative charge get delocalized with a pi bond or we can say pi electrons of the C=O group.
Let’s see the resonance structure of CH3COO−
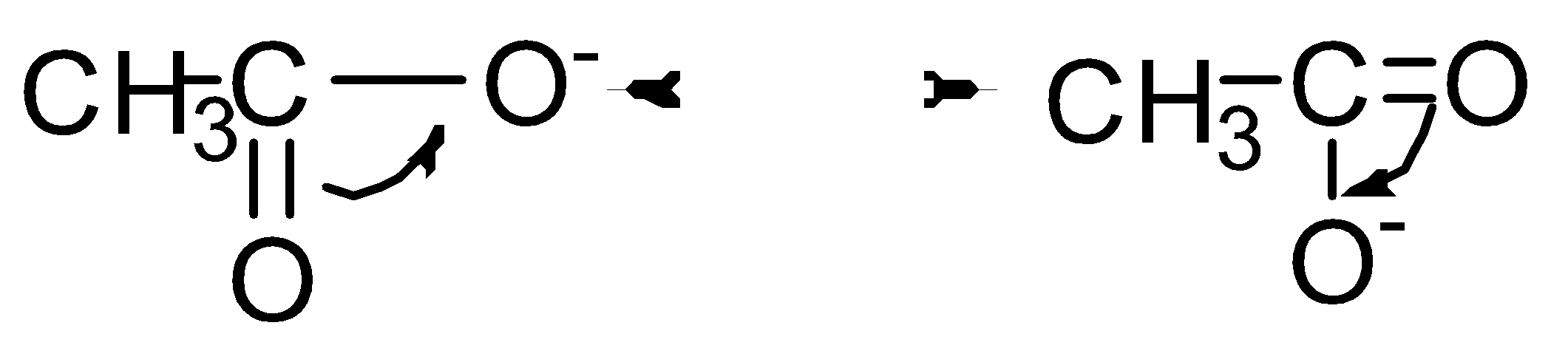
Therefore, CH3COONa has high resonance energy as it shows more resonance.
Similarly, we see compare the resonance energy of other given pairs by observing the delocalization of the pi- electrons
B. CH2=CH− and CH2=CH−OH
Here, oxygen is more electronegative than carbon. So, oxygen pulls the electrons more easily than carbon. Therefore, CH2=CH−OH has more resonance energy than CH2=CH−.
C. 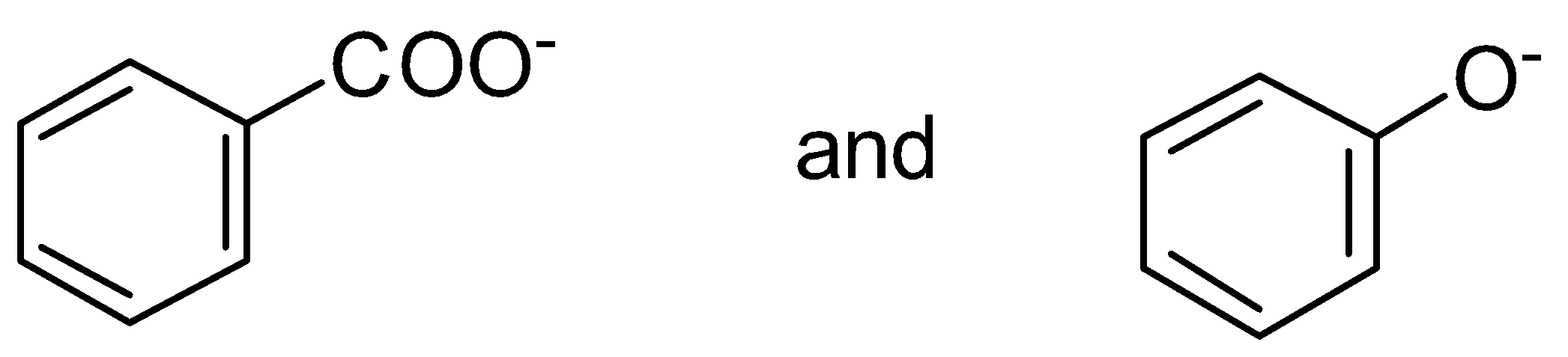
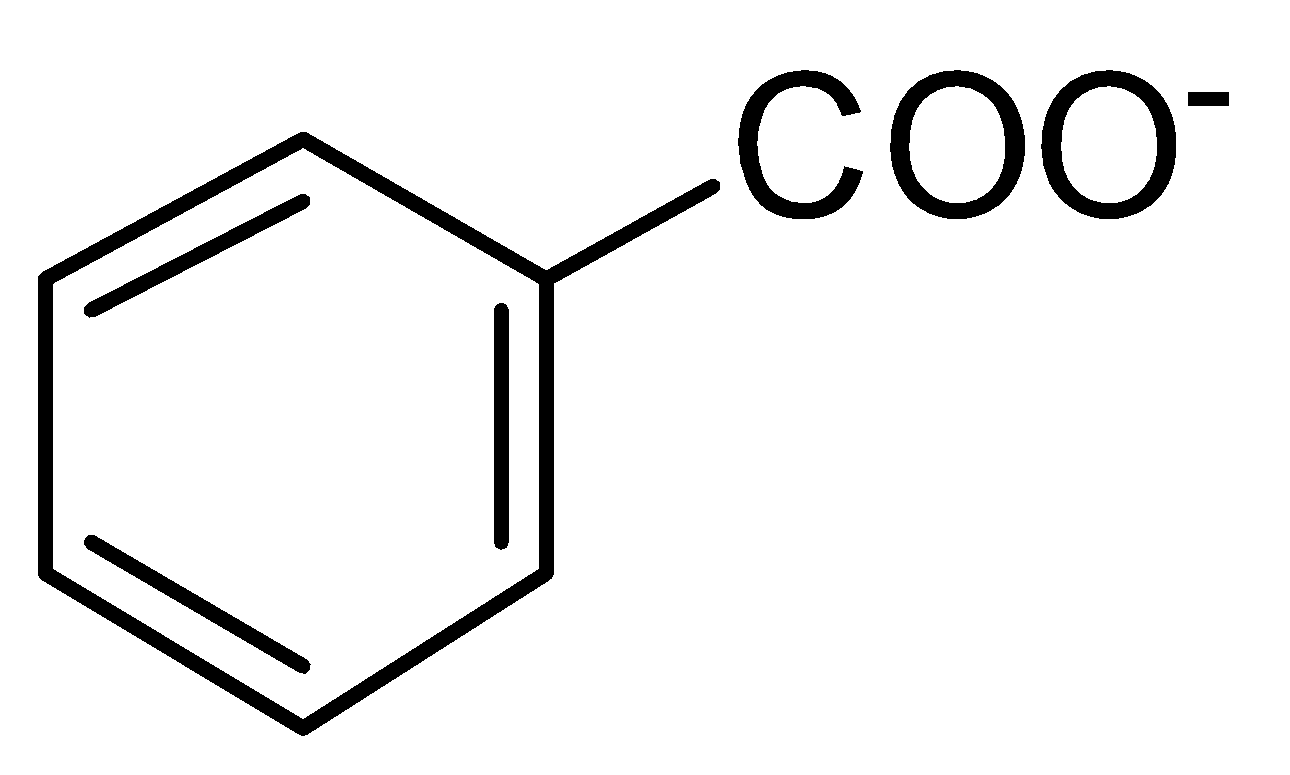 is a more stable resonance. Hence, more resonance energy.
is a more stable resonance. Hence, more resonance energy.
D. 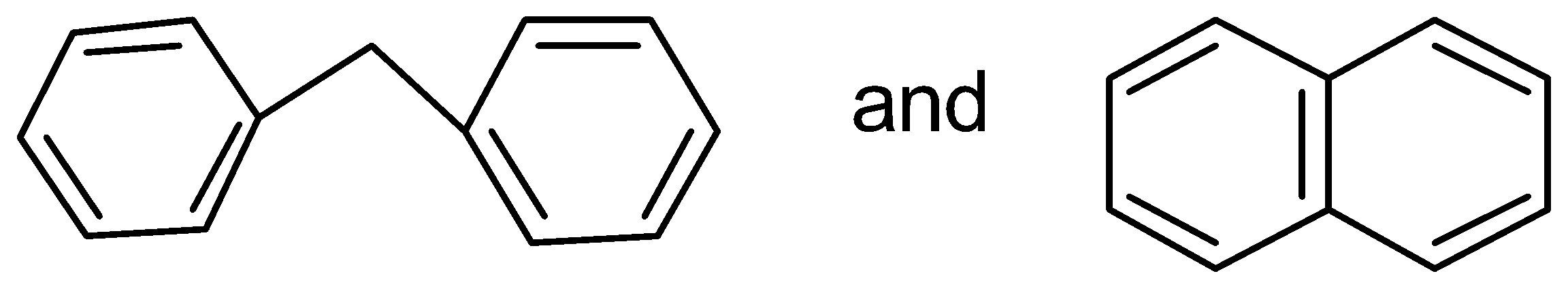
In this case, the two benzene rings which are connected with a common bond show complete resonance in the structure.
Therefore,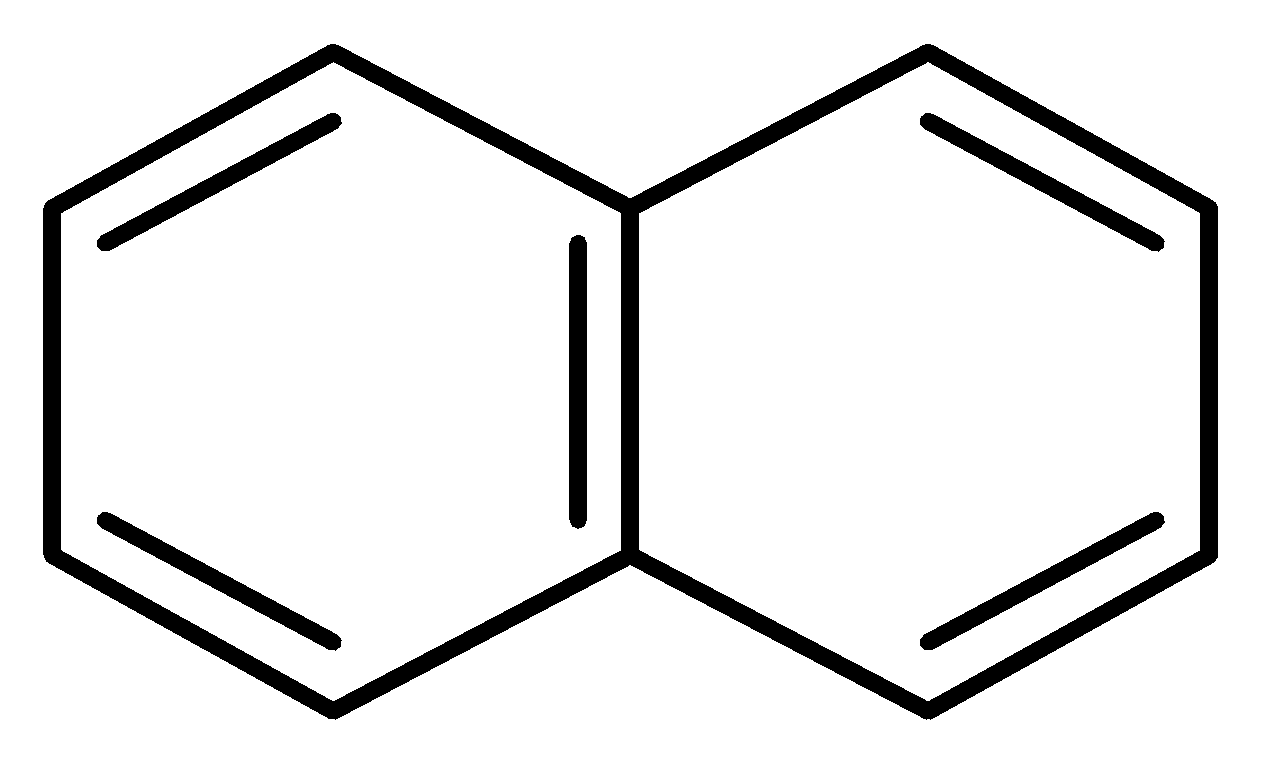 has more resonance energy.
has more resonance energy.
E. 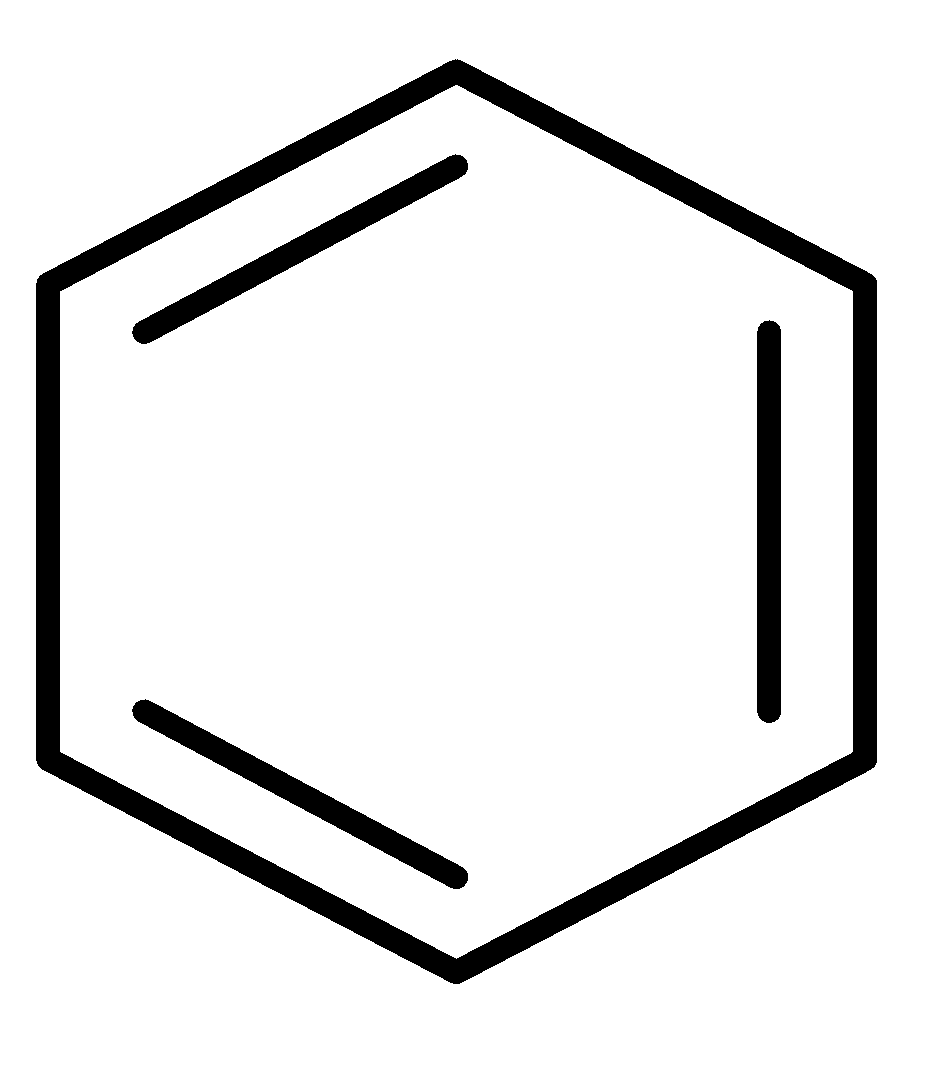 and CH2=CH−CH=CH−CH=CH2
and CH2=CH−CH=CH−CH=CH2
In this pair, the benzene ring is an aromatic compound so it will show more resonance. Hence, it has more resonance energy.
Note:
Resonance energy is also called resonance stabilization energy. This is so because it depends upon the stability and aromaticity of the compounds. Also, it depends upon the electronegativity of the heteroatom like oxygen, nitrogen etc. attached to the organic compound.
