Question
Question: Which of the following has electron deficient compounds? (A) \({B_2}{H_6}\), \(AlC{l_3}\) (B) \(...
Which of the following has electron deficient compounds?
(A) B2H6, AlCl3
(B) C2H6, Al2Cl6
(C) SF2, Cl2O
(D) NaBH4, ICl
Solution
An electron deficient compound is the one in which there is an insufficient number of electrons to complete the octet of the central atom. These compounds contain insufficient numbers of electrons to form normal electron-pair bonds between each pair of the bonded atoms.
Complete step by step solution:
B2H6 (Diborane) and AlCl3 are electron deficient compounds. This is because:
In case of diborane, (as shown in the structure), each boron bonds with 4 hydrogen atoms.
But the valency of boron is 3 and each boron gives 3 valence electrons.
But in case of the 4th bond, there is no electron from boron’s side and hence making it electron deficient.
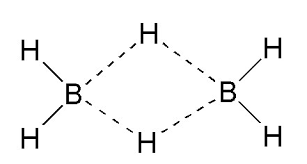
Now,
In case of AlCl3 (as shown), Al atom bonds with 3 Cl atoms. But it violates the octet rule. There are no bonds or lone pairs, which makes the surrounding electron count 8. It has only 3 bonds which makes only 6 electrons and hence making it an electron deficient compound.
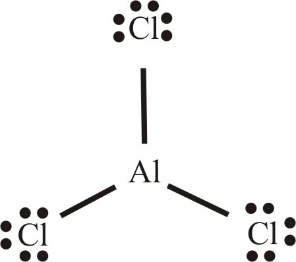
Therefore, B2H6 and AlCl3 both are electron deficient compounds.
Hence, option A is correct.
Note: Diborane is used in rocket propellants. It is also used as a rubber vulcanizer and a catalyst for olefin polymerization whereas aluminium chloride is used in the pharmaceutical industry. It is also used as an antiperspirant in products such as Drysol.
