Question
Question: Which of the following has distorted tetrahedral shape? (A) \(Si{H_4}\) (B) \(CC{l_4}\) (C) \(...
Which of the following has distorted tetrahedral shape?
(A) SiH4
(B) CCl4
(C) CH4
(D) CHCl3
Solution
A carbon atom with four bonds and bond angle of approximately 109.5o is tetrahedral geometry. But when bond angle is different from 109.5o i.e., less than this angle then it is called distorted tetrahedral geometry.
This is due to repulsion between the bond pair and lone pair of electron.
Complete step by step answer:
Let us discuss the geometry of each molecule one by one.
(1) CHCl3 chloroform: The molecular shape of CHCl3 is tetrahedral. It means that H-atoms and three Cl-atoms. The vertices of a triangular based pyramid around the central C-atom.
This is because polar covalent bonds are asymmetrically arranged around the central atom of a molecule.
The geometry of molecules is not regular because the dipole moment of CHCl3 is not zero.
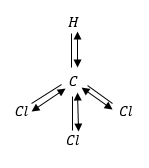
CHCl3 has maximum dipole moment because in tetrahedral structure all Cl-atoms will be at bottom and add to individual dipoles.
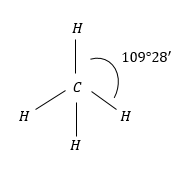
(2) CH4: CH4 molecules have regular tetrahedral geometry due H-atoms present and regular tetrahedron structure.
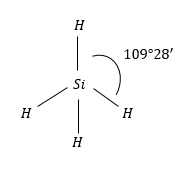
SiH4: Molecular geometry of this molecule is also regular tetrahedral. Because it is surrounded by the same atoms i.e., H-atoms.
Therefore, the same symmetric charge distribution around Siatom and molecule is non-polar.
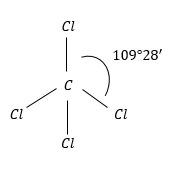
CCl4: This has regular geometry because of the same atoms surrounding the C-atom.
Therefore, the dipole moment of the molecule is zero.
Therefore, from the above explanation the correct option is (D) CHCl3.
Note:
Molecules show regular tetrahedral geometry when the central atom is surrounded by the same atoms. The resulting dipole moment becomes zero.
But when central atoms surrounded by different atoms [differ in their electronegativity] have dipole moment and therefore their geometry is distorted.
