Question
Question: Which of the following has an optical isomer? A) \(\text{ }{{\left[ \text{Zn(en)(N}{{\text{H}}_{\t...
Which of the following has an optical isomer?
A) [Zn(en)(NH3)2]2+
B) [Co(en)3]3+
C) [Co(H2O)4(en)]3+
D) [Zn(en)2]2+ (en = ethylenediamine)
Solution
The isomers which rotate the plane of polarised light equally but in opposite directions are called optically active isomers. Complexes with the type [M(AA)3] , [M(AA)2X2] , [M(AA)2XY] exists as an optical isomer. The complexes which do not possess the plane are said to be optically active. Their mirror images are non-superimposable to each other.
Complete step by step solution:
Stereoisomers are that isomer which has the same position of atoms or group but they differ in the spatial arrangement around the central atom. There are two types of isomerism viz., geometrical isomerism and optical isomerism.
Certain substances can rotate the plane of polarised light. These are called optically active substances. The isomers which rotate the plane of polarised light equally but in opposite directions are called optically active isomers.
The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in structure.
Complexes with the type [M(AA)3] exist as an optical isomer. Here, M is the metal atom and (AA) is a bidentate ligand. The bidentate ligand is a ligand that has to go to the donor site. It donates its electron from these donor sites and forms two bonds with metal.
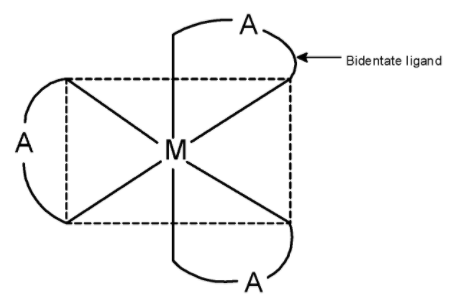
The ethane-1, 2-diamine abbreviated as ‘en’ has two nitrogen atoms with an electron pair. Thus, these nitrogen atoms bond to the metal atom.
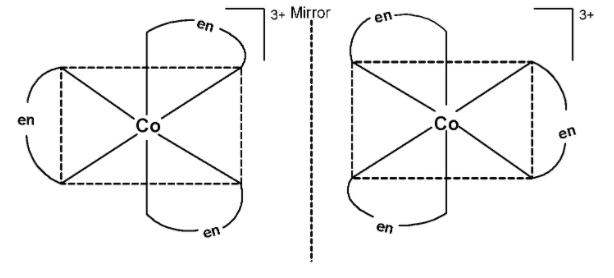
Generally, cobalt forms 6 bonds with the ligands. Since ethane-1, 2-diamine has two donor sites, three ‘en’ ligands are surrounding the cobalt metal. The three ligands are placed as shown below.
The plane of symmetry does not pass through the complex. Thus, [CO(en)3]3+ exhibits optical activity. the optical isomers of the complex are as shown below,
The [Zn(en)(NH3)2]2+ , [Co(H2O)4(en)]3+ , [Zn(en)2]2+ possess the plane of symmetry which divides the molecule such the one half is a mirror image of other. Thus these do not exhibit optical isomerism.
Thus, [CO(en)3]3+ has optical isomers.
Hence, (B) is the correct option.
Note: Another example of the [M(AA)3] is [Cr(ox)3]3− , where ‘ox’ is an oxalate ion or oxalato ligand. It has two donor oxygen atoms. Note that, here the three ligands visually acquire the three corners of the triangle thus the imaginary plane if passed through the complex does not cut it into equal halves.
