Question
Chemistry Question on Hybridisation
Which of the following has a square pyramidal shape?
A
PCI5
B
BrF5
C
PF5
D
[Ni(CN)4]2-
Answer
BrF5
Explanation
Solution
BrF5 (Bromine pentafluoride) has a square pyramidal shape. This is because it has five regions of electron density around the central bromine atom, with one being a lone pair of electrons, resulting in a square pyramidal molecular geometry.
BrF5 has 1 lone pair and 5 bond. Therefore, geometry is octahedral, shape is square pyramidal.
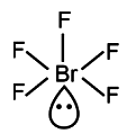
So, the correct option is (B): BrF5
