Question
Question: Which of the following has a square planar structure? [A] \(N{{H}_{4}}^{+}\) [B] \(B{{F}_{4}}\)...
Which of the following has a square planar structure?
[A] NH4+
[B] BF4
[C] XeF4
[D] CCl4
Solution
HINT: To find the geometry of any complex, we need to determine the coordination number of the complex. If we know the coordination number, we can easily find out the hybridization and the geometry of the complex.
COMPLETE STEP BY STEP SOLUTION: Let us discuss each option to find out if they are in square planar geometry or not-
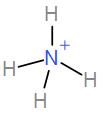
In the first option we have NH4+
Number of valence electrons in nitrogen = 5.
From the hydrogen atoms we have a total contribution of 4.
Therefore, the total number of electrons is 9.
It has a positive overall charge so we will subtract 1 from 9. Therefore, the total number of electrons is 8.
Dividing it by 2 will give us the coordination number and which will give us the hybridisation and shape.
The coordination number is 4. Therefore, the hybridisation is sp3 and shape is tetrahedral. Therefore, this is not the correct answer.
In the next option we have BF4.
Valence electrons in boron are 3 and contribution for each fluorine atom is 1.
Therefore, the total number of electrons is 7.
Now as we can see it is not even so if we consider it as a cation or anion then the number of electrons will still be 6 or 8. Thus we will get a coordination number of either 3 or 4. So, this cannot be of square planar shape either.
Then we have XeF4.
Number of valence electrons in xenon is 8 and contribution from each fluorine atom is 4 which will give us a total number of electrons of 12. Therefore, the coordination number will be 6. So, the shape is octahedral and the hybridisation is sp3d2 .
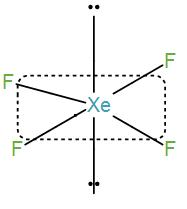
However, there are only four fluorine atoms so the remaining 4 electrons will exist as lone pairs and thus we will get a square planar structure here.
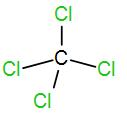
And lastly we have CCl4.
Number of valence electron in carbon is 4 and each chlorine atom contributes 1 electron. So, the total electron will be 8 and thus coordination number is 4. Therefore, the shape is tetrahedral and the hybridisation is sp3.
We can see from the above discussion that XeF4 has a square planar structure.
Therefore, the correct answer is option [C] XeF4.
NOTE: We have used VSEPR theory above to determine the shape and hybridisation of the given compounds. Although VSEPR is quite successful in determining the geometries of molecules, there are certain drawbacks to this theory. This theory fails to determine the shape of isoelectronic species. It also does not take the relative size of the constituents under consideration. It is unable to explain the atomic orbital overlaps.
