Question
Question: Which of the following has a non-zero dipole moment? (a)- \(PC{{l}_{5}}\) (b)- \(Cl{{F}_{3}}\) ...
Which of the following has a non-zero dipole moment?
(a)- PCl5
(b)- ClF3
(c)- XeF4
(d)- C2H5C≡CC2H5
Solution
Dipole moment tells the charge separation between two atoms and if the structure is symmetrical structure then the dipole moment will be zero because all the dipole moments are canceled out. For the compound to have a non-zero dipole moment then the compound must have an asymmetrical structure.
Complete step by step answer:
Dipole moment tells the charge separation between two atoms and if the structure is a symmetrical structure then the dipole moment will be zero because all the dipole moments are canceled out. Let us see the structures of all the compounds one by one:
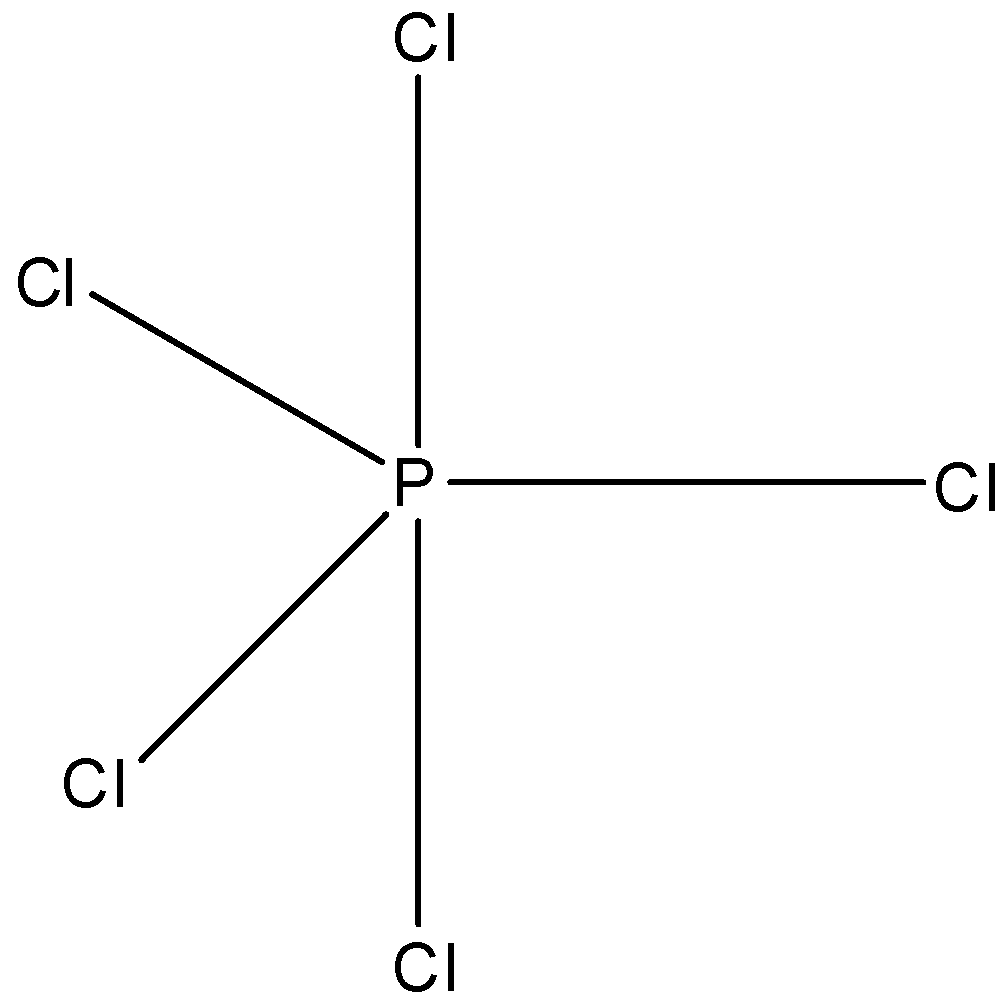
(a)- PCl5: This compound is phosphorus pentachloride. The structure of this compound is trigonal bipyramidal and this is a symmetrical structure, so the net dipole moment will be zero. The structure is given below:
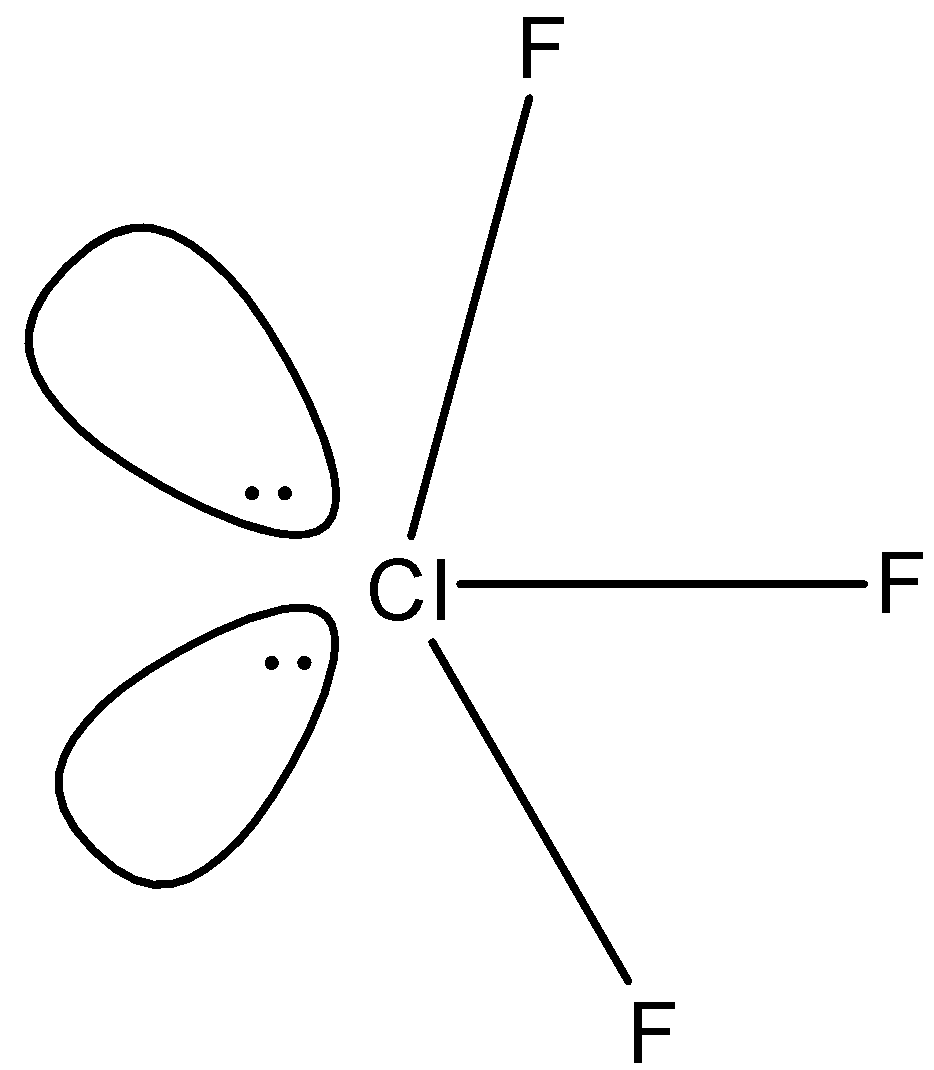
(b)- ClF3 : This compound is chlorine trifluoride. There are three fluorine atoms attached with the chlorine and there are two lone pairs on the chlorine atom due to which the shape becomes distorted and the net-dipole is non-zero. The structure is given below:
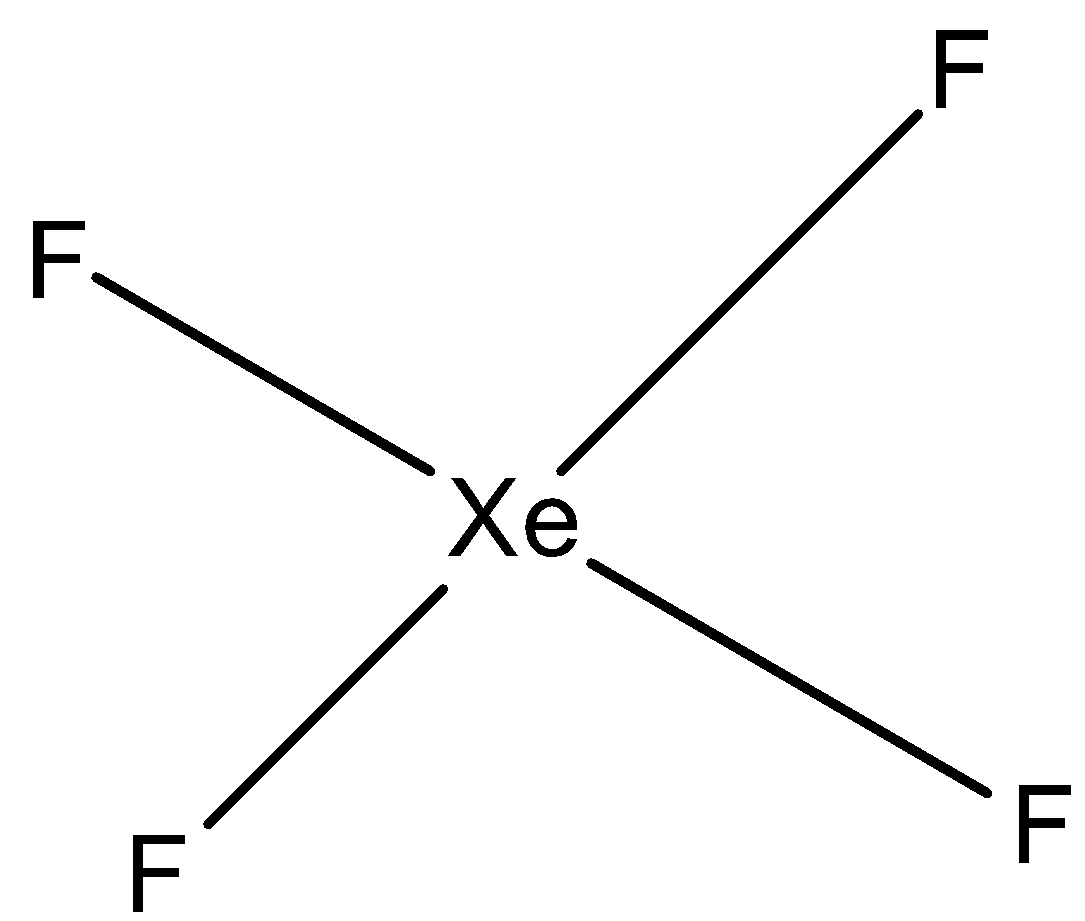
(c)- XeF4: This compound is xenon tetrafluoride. The structure of this compound is square planar and this is a symmetrical structure. So, the net dipole is zero. The structure is given below:
(d)- C2H5C≡CC2H5: This compound is hexan-3-yne. The structure of the compound is linear and the linear structure is a symmetrical structure. The dipole will be zero.
C2H5−C≡C−C2H5 The correct option is option “D” .
Note: All the compounds having a linear structure will always have zero dipole moment. The dipole moment is denoted by the symbol μ and pronounced as meu. The unit is debye and written as D.
